A Relative Bioavailability Study of Two Misoprostol Formulations Following a Single Oral or Sublingual Administration
- PMID: 32116725
- PMCID: PMC7029744
- DOI: 10.3389/fphar.2020.00050
A Relative Bioavailability Study of Two Misoprostol Formulations Following a Single Oral or Sublingual Administration
Abstract
Introduction: Misoprostol (Cytotec) was primarily made for treating gastric ulcers. However today it is mostly used for abortion, treating postpartum hemorrhage, and for induction of labor. The tablet contains 200 µg of misoprostol, yet the dosages used for induction of labor are much smaller (25-50 µg), leading to uncertainty of dosage in daily use.
Aim: To evaluate and compare the relative bioavailability of two misoprostol products (Angusta 25 µg and Cytotec 200 µg tablets) administered orally or sublingually given in a daily clinical setting to women admitted for induction of labor at term.
Methods: Women carrying a live, singleton fetus in a cephalic position and with a gestational age between 259 and 296 days were included. Blood samples were collected at 0, 5, 10, 20, 30, 40, 50, 75, 100, 120, 180, and 240 minutes. A serum analytical assay was performed and pharmacokinetic parameters were calculated. Patients were assigned to one of three groups.
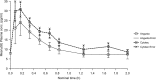
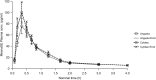
Results: A total of 72 patients were included. No significant differences demographic characteristics were found. The ratios for AUC, AUC (0-t), and Cmax were similar in all three groups, but CI-values were outside the required 80-125%. Sublingual administration yielded a 20-30% higher bioavailability and a 50% higher Cmax than compared to the oral route.
Conclusion: The relative bioavailability between Angusta and Cytotec could not be confirmed as being equal at the 25 µg or 50 µg level because the 90% CI-values when comparing the ratios for AUC, AUC(0-t), and Cmax were wider than accepted. The reason for this could be the real-life, non-standardized circumstances in which the study was conducted. Sublingual administration seems to have higher bioavailability than oral administration. More studies are needed to ascertain an optimal dosage regime balancing both safety and efficacy for mother and child.
Clinical trial registration: www.ClinicalTrials.gov, identifier NCT02516631.
Keywords: human; induction of labor; misoprostol; pharmacokinetics; pregnancy.
Copyright © 2020 Amini, Reis and Wide-Swensson.
Figures

References
-
- Berard V., Fiala C., Cameron S., Bombas T., Parachini M., Gemzell-Danielsson K., et al. (2014). Instability of misoprostol tablets stored outside the blister: a potential serious concern for clinical outcome in medical abortion. PloS One 9 (12), e112401. 10.1371/journal.pone.0112401 - DOI - PMC - PubMed
-
- Bioequivalence Task Force (1988). Report on recommendations from the bioequivalence hearing conducted by the Food and Drug Administration.
-
- Center for Drug Evaluation and Research; US Food and Drug Administration (1992). Guidance: Statistical Procedures for Bioequivalence Studies Using a Standard Two-Treatment Crossover Design [cited 2018 01-01]; Available from: https://www.fda.gov/media/70958/download.
Associated data
LinkOut - more resources
Full Text Sources
Medical

