Molecular Dynamics of Lipopolysaccharide-Induced Lung Injury in Rodents
- PMID: 32116752
- PMCID: PMC7012903
- DOI: 10.3389/fphys.2020.00036
Molecular Dynamics of Lipopolysaccharide-Induced Lung Injury in Rodents
Abstract
Acute respiratory distress syndrome (ARDS) is a common disease entity in critical care medicine and is still associated with a high mortality. Because of the heterogeneous character of ARDS, animal models are an insturment to study pathology in relatively standardized conditions. Rodent models can bridge the gap from in vitro investigations to large animal and clinical trials by facilitating large sample sizes under physiological conditions at comparatively low costs. One of the most commonly used rodent models of acute lung inflammation and ARDS is administration of lipopolysaccharide (LPS), either into the airways (direct, pulmonary insult) or systemically (indirect, extra-pulmonary insult). This narrative review discusses the dynamics of important pathophysiological pathways contributing to the physiological response to LPS-induced injury. Pathophysiological pathways of LPS-induced lung injury are not only influenced by the type of the primary insult (e.g., pulmonary or extra-pulmonary) and presence of additional stimuli (e.g., mechanical ventilation), but also by time. As such, findings in animal models of LPS-induced lung injury may depend on the time point at which samples are obtained and physiological data are captured. This review summarizes the current evidence and highlights uncertainties on the molecular dynamics of LPS-induced lung injury in rodent models, encouraging researchers to take accurate timing of LPS-induced injury into account when designing experimental trials.
Keywords: acute respiratory distress syndrome; dynamics; inflammation; lipopolysaccharide-induced lung injury; time-dependent; toll-like receptor 4.
Copyright © 2020 Domscheit, Hegeman, Carvalho and Spieth.
Figures
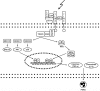

References
-
- Bersten A. D., Edibam C., Hunt T., Moran J., Australian and New Zealand Intensive Care Society Clinical Trials Group (2002). Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am. J. Respir. Crit. Care Med. 165, 443–448. 10.1164/ajrccm.165.4.2101124, PMID: - DOI - PubMed
-
- Bitterman P. B. (1992). Pathogenesis of fibrosis in acute lung injury. Am. J. Med. 92, 39S–43S. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

