Potential Elimination of Human Gut Resistome by Exploiting the Benefits of Functional Foods
- PMID: 32117102
- PMCID: PMC7026006
- DOI: 10.3389/fmicb.2020.00050
Potential Elimination of Human Gut Resistome by Exploiting the Benefits of Functional Foods
Abstract
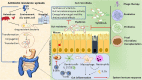
Recent advances in technology over the last decades have strived to elucidate the diverse and abundant ecosystem of the human microbiome. The intestinal microbiota represents a densely inhabited environment that offers a plethora of beneficial effects to the host's wellbeing. On the other hand, it can serve as a potential reservoir of Multi-Drug Resistant (MDR) bacteria and their antibiotic-resistant genes (ARgenes), which comprise the "gut resistome." ARgenes, like antibiotics, have been omnipresent in the environment for billions of years. In the context of the gut microbiome, these genes may conflate into exogenous MDR or emerge in commensals due to mutations or gene transfers. It is currently generally accepted that Antimicrobial Resistance (AMR) poses a serious threat to public health worldwide. It is of paramount importance that researchers focus on, amongst other parameters, elaborating strategies to manage the gut resistome, particularly focusing on the diminution of AMR. Potential interventions in the gut microbiome field by Fecal Microbiota Transplant (FMT) or functional foods are newly emerged candidates for the uprooting of MDR strains and restoring dysbiosis and resilience. Probiotic nutrition is thought to diminish gut colonization from pathobionts. Yet only a few studies have explored the effects of antibiotics use on the reservoir of AR genes and the demanding time for return to normal by gut microbiota-targeted strategies. Regular administration of probiotic bacteria has recently been linked to restoration of the gut ecosystem and decrease of the gut resistome and AR genes carriers. This review summarizes the latest information about the intestinal resistome and the intriguing methods of fighting against AMR through probiotic-based methods and gut microbial shifts that have been proposed. This study contains some key messages: (1) AMR currently poses a lethal threat to global health, and it is pivotal for the scientific community to do its utmost in fighting against it; (2) human gut microbiome research, within the last decade especially, seems to be preoccupied with the interface of numerous diseases and identifying a potential target for a variety of interventions; (3) the gut resistome, comprised of AR genesis, presents very early on in life and is prone to shifts due to the use of antibiotics or dietary supplements; and (4) future strategies involving functional foods seem promising for the battle against AMR through intestinal resistome diminution.
Keywords: antimicrobial resistance; antimicrobial resistance genes; gut microbiome; prebiotics; probiotics; resistome.
Copyright © 2020 Tsigalou, Konstantinidis, Stavropoulou, Bezirtzoglou and Tsakris.
Figures
Similar articles
-
Potential influence of antimicrobial resistance gene content in probiotic bacteria on the gut resistome ecosystems.Front Nutr. 2023 Feb 1;10:1054555. doi: 10.3389/fnut.2023.1054555. eCollection 2023. Front Nutr. 2023. PMID: 36819705 Free PMC article. Review.
-
Exploring the role of gut microbiota in antibiotic resistance and prevention.Ann Med. 2025 Dec;57(1):2478317. doi: 10.1080/07853890.2025.2478317. Epub 2025 Mar 17. Ann Med. 2025. PMID: 40096354 Free PMC article. Review.
-
Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner.Nat Microbiol. 2021 Aug;6(8):1043-1054. doi: 10.1038/s41564-021-00920-0. Epub 2021 Jul 5. Nat Microbiol. 2021. PMID: 34226711 Free PMC article.
-
Good microbes, bad genes? The dissemination of antimicrobial resistance in the human microbiome.Gut Microbes. 2022 Jan-Dec;14(1):2055944. doi: 10.1080/19490976.2022.2055944. Gut Microbes. 2022. PMID: 35332832 Free PMC article. Review.
-
The human microbiome as a reservoir of antimicrobial resistance.Front Microbiol. 2013 Apr 17;4:87. doi: 10.3389/fmicb.2013.00087. eCollection 2013. Front Microbiol. 2013. PMID: 23616784 Free PMC article.
Cited by
-
Fecal Microbiome and Resistome Profiling of Healthy and Diseased Pakistani Individuals Using Next-Generation Sequencing.Microorganisms. 2021 Mar 17;9(3):616. doi: 10.3390/microorganisms9030616. Microorganisms. 2021. PMID: 33802711 Free PMC article.
-
Characterization of the Composition Variation of Healthy Human Gut Microbiome in Correlation with Antibiotic Usage and Yogurt Consumption.Antibiotics (Basel). 2022 Dec 16;11(12):1827. doi: 10.3390/antibiotics11121827. Antibiotics (Basel). 2022. PMID: 36551483 Free PMC article.
-
Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota With Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces.Front Public Health. 2021 Mar 8;8:578089. doi: 10.3389/fpubh.2020.578089. eCollection 2020. Front Public Health. 2021. PMID: 33763399 Free PMC article. Clinical Trial.
-
Antibiotics and probiotics impact gut antimicrobial resistance gene reservoir in COVID-19 patients.Gut Microbes. 2022 Jan-Dec;14(1):2128603. doi: 10.1080/19490976.2022.2128603. Gut Microbes. 2022. PMID: 36201636 Free PMC article.
-
Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives.Microorganisms. 2021 Sep 27;9(10):2041. doi: 10.3390/microorganisms9102041. Microorganisms. 2021. PMID: 34683362 Free PMC article. Review.
References
-
- Al-Kharousi Z., Guizani N., Al-Sadi A., Al Bulushi I. (2018). “Fresh fruit and vegetable bacteria: Diversity, antibiotic resistance and their possible contribution to gut microbiota,” in Fruit and Vegetable Consumption and Health: New Research, ed. Jongen W. (Hauppauge, NY: Nova Science Publishers, Inc; ), 39–66.
-
- Angelakis E., Raoult D. (2018). Gut microbiota modifications and weight gain in early life. Hum. Microbio. J. 7 10–14. 10.1016/j.humic.2018.01.002 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

