Finegoldia magna, an Anaerobic Gram-Positive Bacterium of the Normal Human Microbiota, Induces Inflammation by Activating Neutrophils
- PMID: 32117109
- PMCID: PMC7025542
- DOI: 10.3389/fmicb.2020.00065
Finegoldia magna, an Anaerobic Gram-Positive Bacterium of the Normal Human Microbiota, Induces Inflammation by Activating Neutrophils
Abstract
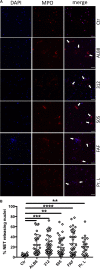
The Gram-positive anaerobic commensal Finegoldia magna colonizes the skin and other non-sterile body surfaces, and is an important opportunistic pathogen. Here we analyzed the effect of F. magna on human primary neutrophils. F. magna strains ALB8 (expressing protein FAF), 312 (expressing protein L) and 505 (naturally lacking both protein FAF and L) as well as their associated proteins activate neutrophils to release reactive oxygen species, an indication for neutrophil oxidative burst. Co-incubation of neutrophils with the bacteria leads to a strong increase of CD66b surface expression, another indicator for neutrophil activation. Furthermore, all tested stimuli triggered the release of NETs from the activated neutrophils, pointing to a host defense mechanism in response to the tested stimuli. This phenotype is dependent on actin rearrangement, NADPH oxidases and the ERK1/2 pathway. Proteins FAF and L also induced the secretion of several pro-inflammatory neutrophil proteins; HBP, IL-8 and INFγ. This study shows for the first time a direct interaction of F. magna with human neutrophils and suggests that the activation of neutrophils plays a role in F. magna pathogenesis.
Keywords: CD66b expression; GPAC; NETs; anaerobic Gram-positive cocci; host-pathogen interactions; inflammation; neutrophils.
Copyright © 2020 Neumann, Björck and Frick.
Figures



References
-
- Akerstrom B., Bjorck L. (1989). Protein L: an immunoglobulin light chain-binding bacterial protein. Characterization of binding and physicochemical properties. J. Biol. Chem. 264 19740–19746. - PubMed
-
- Anderson A. L., Zheng Y., Song D., Larosa D., Van Rooijen N., Kierstein G., et al. (2012). The B-cell superantigen Finegoldia magna protein L causes pulmonary inflammation by a mechanism dependent on MyD88 but not B cells or immunoglobulins. Inflamm. Res. 61 161–169. 10.1007/s00011-012-0436-8 - DOI - PMC - PubMed
-
- Bjorck L. (1988). Protein L. A novel bacterial cell wall protein with affinity for Ig L chains. J. Immunol. 140 1194–1197. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

