Heavy Metal Toxicity in Armed Conflicts Potentiates AMR in A. baumannii by Selecting for Antibiotic and Heavy Metal Co-resistance Mechanisms
- PMID: 32117111
- PMCID: PMC7008767
- DOI: 10.3389/fmicb.2020.00068
Heavy Metal Toxicity in Armed Conflicts Potentiates AMR in A. baumannii by Selecting for Antibiotic and Heavy Metal Co-resistance Mechanisms
Abstract
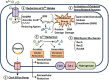
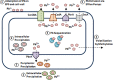
Acinetobacter baumannii has become increasingly resistant to leading antimicrobial agents since the 1970s. Increased resistance appears linked to armed conflicts, notably since widespread media stories amplified clinical reports in the wake of the American invasion of Iraq in 2003. Antimicrobial resistance is usually assumed to arise through selection pressure exerted by antimicrobial treatment, particularly where treatment is inadequate, as in the case of low dosing, substandard antimicrobial agents, or shortened treatment course. Recently attention has focused on an emerging pathogen, multi-drug resistant A. baumannii (MDRAb). MDRAb gained media attention after being identified in American soldiers returning from Iraq and treated in US military facilities, where it was termed "Iraqibacter." However, MDRAb is strongly associated in the literature with war injuries that are heavily contaminated by both environmental debris and shrapnel from weapons. Both may harbor substantial amounts of toxic heavy metals. Interestingly, heavy metals are known to also select for antimicrobial resistance. In this review we highlight the potential causes of antimicrobial resistance by heavy metals, with a focus on its emergence in A. baumanni in war zones.
Keywords: Acinetobacter baumannii; antimicrobial resistance; bacteria; conflict; heavy metal tolerance; heavy metals; weapons.
Copyright © 2020 Bazzi, Abou Fayad, Nasser, Haraoui, Dewachi, Abou-Sitta, Nguyen, Abara, Karah, Landecker, Knapp, McEvoy, Zaman, Higgins and Matar.
Figures
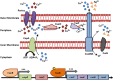
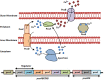
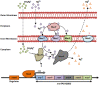
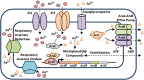
References
-
- Abadin H., Ashizawa A., Stevens Y.-W., ATSDR, Llados F., Diamond G., et al. (2007). Toxic Substances Portal- Lead. Atlanta: Agency for Toxic Substances and Disease Registry; Available at: https://www.ncbi.nlm.nih.gov/pubmed/24049859 - PubMed
-
- Abbas S. Z., Rafatullah M., Hossain K., Ismail N., Tajarudin H. A., Abdul Khalil H. P. S. (2017). A review on mechanism and future perspectives of cadmium-resistant bacteria. Int. J. Environ. Sci. Technol. 15 243–262. 10.1007/s13762-017-1400-5 - DOI

