A Novel Polyester Hydrolase From the Marine Bacterium Pseudomonas aestusnigri - Structural and Functional Insights
- PMID: 32117139
- PMCID: PMC7031157
- DOI: 10.3389/fmicb.2020.00114
A Novel Polyester Hydrolase From the Marine Bacterium Pseudomonas aestusnigri - Structural and Functional Insights
Abstract
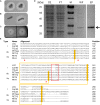
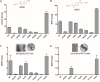
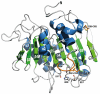
Biodegradation of synthetic polymers, in particular polyethylene terephthalate (PET), is of great importance, since environmental pollution with PET and other plastics has become a severe global problem. Here, we report on the polyester degrading ability of a novel carboxylic ester hydrolase identified in the genome of the marine hydrocarbonoclastic bacterium Pseudomonas aestusnigri VGXO14 T . The enzyme, designated PE-H, belongs to the type IIa family of PET hydrolytic enzymes as indicated by amino acid sequence homology. It was produced in Escherichia coli, purified and its crystal structure was solved at 1.09 Å resolution representing the first structure of a type IIa PET hydrolytic enzyme. The structure shows a typical α/β-hydrolase fold and high structural homology to known polyester hydrolases. PET hydrolysis was detected at 30°C with amorphous PET film (PETa), but not with PET film from a commercial PET bottle (PETb). A rational mutagenesis study to improve the PET degrading potential of PE-H yielded variant PE-H (Y250S) which showed improved activity, ultimately also allowing the hydrolysis of PETb. The crystal structure of this variant solved at 1.35 Å resolution allowed to rationalize the improvement of enzymatic activity. A PET oligomer binding model was proposed by molecular docking computations. Our results indicate a significant potential of the marine bacterium P. aestusnigri for PET degradation.
Keywords: PET; Pseudomonas aestusnigri; crystal structure; marine bacteria; polyester degradation; polyethylene terephthalate.
Copyright © 2020 Bollinger, Thies, Knieps-Grünhagen, Gertzen, Kobus, Höppner, Ferrer, Gohlke, Smits and Jaeger.
Figures




References
-
- Barth M., Oeser T., Wei R., Then J., Schmidt J., Zimmermann W. (2015). Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 93 222–228. 10.1016/J.BEJ.2014.10.012 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

