KSHV: Immune Modulation and Immunotherapy
- PMID: 32117196
- PMCID: PMC7025529
- DOI: 10.3389/fimmu.2019.03084
KSHV: Immune Modulation and Immunotherapy
Abstract
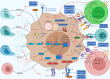
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) is associated with KS, primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD). To ensure its own survival and propagation, KSHV employs an extensive network of viral proteins to subvert the host immune system, resulting in lifelong latent infection. Modulation of cellular and systemic immune defenses allows KSHV to persist in the host, which may eventually lead to the progression of KSHV-associated cancers. Due to KSHV's reliance on modifying immune responses to efficiently infect its host, immunotherapy is an attractive option for treating KSHV-associated malignancies. In this review, we will focus on the mechanisms by which KSHV evades the immune system and the current immune-related clinical strategies to treat KSHV-associated disease.
Keywords: KSHV; cytokines; immune system modulation; immunotherapy; interferon; oncoviruses.
Copyright © 2020 Broussard and Damania.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

