Pleiotropic Role and Bidirectional Immunomodulation of Innate Lymphoid Cells in Cancer
- PMID: 32117199
- PMCID: PMC7010811
- DOI: 10.3389/fimmu.2019.03111
Pleiotropic Role and Bidirectional Immunomodulation of Innate Lymphoid Cells in Cancer
Abstract
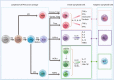
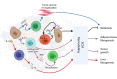
Innate lymphoid cells (ILCs) are largely tissue resident and respond rapidly toward the environmental signals from surrounding tissues and other immune cells. The pleiotropic function of ILCs in diverse contexts underpins its importance in the innate arm of immune system in human health and disease. ILCs derive from common lymphoid progenitors but lack adaptive antigen receptors and functionally act as the innate counterpart to T-cell subsets. The classification of different subtypes is based on their distinct transcription factor requirement for development as well as signature cytokines that they produce. The discovery and subsequent characterization of ILCs over the past decade have mainly focused on the regulation of inflammation, tissue remodeling, and homeostasis, whereas the understanding of the multiple roles and mechanisms of ILCs in cancer is still limited. Emerging evidence of the potent immunomodulatory properties of ILCs in early host defense signifies a major advance in the use of ILCs as promising targets in cancer immunotherapy. In this review, we will decipher the non-exclusive roles of ILCs associated with both protumor and antitumor activities. We will also dissect the heterogeneity, plasticity, genetic evidence, and dysregulation in different cancer contexts, providing a comprehensive understanding of the complexity and diversity. These will have implications for the therapeutic targeting in cancer.
Keywords: ILCs; cancer; heterogenity; immunosurveillance; immunothearpy; plasticicity; tumor immune microenvironment.
Copyright © 2020 An, Flores-Borja, Irshad, Deng and Ng.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

