Crucial Involvement of IL-6 in Thrombus Resolution in Mice via Macrophage Recruitment and the Induction of Proteolytic Enzymes
- PMID: 32117207
- PMCID: PMC7019028
- DOI: 10.3389/fimmu.2019.03150
Crucial Involvement of IL-6 in Thrombus Resolution in Mice via Macrophage Recruitment and the Induction of Proteolytic Enzymes
Abstract
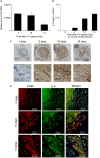
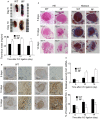
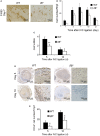
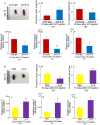
After the ligation of the inferior vena cava (IVC) of wild-type (WT) mice, venous thrombi formed and grew progressively until 5 days and resolved thereafter. Concomitantly, intrathrombotic gene expression of Il6 was enhanced later than 5 days after IVC ligation. IL-6 protein expression was detected mainly in F4/80-positive macrophages in thrombus. When Il6-deficient (Il6-/-) mice were treated in the same manner, thrombus mass was significantly larger than in WT mice. Moreover, the recovery of thrombosed IVC blood flow was markedly delayed in Il6-/- compared with WT mice. F4/80-positive macrophages in thrombus expressed proteolytic enzymes such as matrix metalloproteinase (Mmp) 2, Mmp9, and urokinase-type plasminogen activator (Plau); and their mRNA expression was significantly reduced in Il6-/- mice. Consistently, the administration of anti-IL-6 antibody delayed the thrombus resolution in WT mice, whereas IL-6 administration accelerated thrombus resolution in WT and Il6-/- mice. Moreover, IL-6 in vitro enhanced Mmp2, Mmp9, and Plau mRNA expression in WT-derived peritoneal macrophages in a dose-dependent manner; and the enhancement was abrogated by a specific Stat3 inhibitor, Stattic. Thus, IL-6/Stat3 signaling pathway can promote thrombus resolution by enhancing Mmp2, Mmp9, and Plau expression in macrophages.
Keywords: IL-6; macrophages; matrix metalloproteinases; proteolytic enzymes; thrombosis.
Copyright © 2020 Nosaka, Ishida, Kimura, Kuninaka, Taruya, Ozaki, Tanaka, Mukaida and Kondo.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

