Citrullination Alters the Antiviral and Immunomodulatory Activities of the Human Cathelicidin LL-37 During Rhinovirus Infection
- PMID: 32117246
- PMCID: PMC7010803
- DOI: 10.3389/fimmu.2020.00085
Citrullination Alters the Antiviral and Immunomodulatory Activities of the Human Cathelicidin LL-37 During Rhinovirus Infection
Abstract
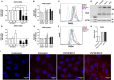
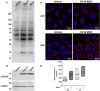
Human rhinoviruses (HRV) are the most common cause of viral respiratory tract infections. While normally mild and self-limiting in healthy adults, HRV infections are associated with bronchiolitis in infants, pneumonia in immunocompromised patients, and exacerbations of asthma and COPD. The human cathelicidin LL-37 is a host defense peptide (HDP) with broad immunomodulatory and antimicrobial activities that has direct antiviral effects against HRV. However, LL-37 is known to be susceptible to the enzymatic activity of peptidyl arginine deiminases (PAD), and exposure of the peptide to these enzymes results in the conversion of positively charged arginines to neutral citrullines (citrullination). Here, we demonstrate that citrullination of LL-37 reduced its direct antiviral activity against HRV. Furthermore, while the anti-rhinovirus activity of LL-37 results in dampened epithelial cell inflammatory responses, citrullination of the peptide, and a loss in antiviral activity, ameliorates this effect. This study also demonstrates that HRV infection upregulates PAD2 protein expression, and increases levels of protein citrullination, including histone H3, in human bronchial epithelial cells. Increased PADI gene expression and HDP citrullination during infection may represent a novel viral evasion mechanism, likely applicable to a wide range of pathogens, and should therefore be considered in the design of therapeutic peptide derivatives.
Keywords: LL-37; cathelicidin; citrullination; inflammation; peptide; rhinovirus; virus.
Copyright © 2020 Casanova, Sousa, Shakamuri, Svoboda, Buch, D'Acremont, Christophorou, Pohl, Stevens and Barlow.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

