Microbial Regulation of Enteric Eosinophils and Its Impact on Tissue Remodeling and Th2 Immunity
- PMID: 32117293
- PMCID: PMC7033414
- DOI: 10.3389/fimmu.2020.00155
Microbial Regulation of Enteric Eosinophils and Its Impact on Tissue Remodeling and Th2 Immunity
Abstract
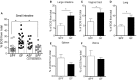
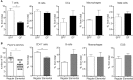
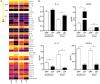
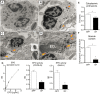
Eosinophils have emerged as multifaceted cells that contribute to tissue homeostasis. However, the impact of the microbiota on their frequency and function at mucosal sites remains unclear. Here, we investigated the role of the microbiota in the regulation of enteric eosinophils. We found that small intestinal (SI) eosinophilia was significantly greater in germ-free (GF) mice compared to specific pathogen free (SPF) controls. This was associated with changes in the production of enteric signals that regulate eosinophil attraction and survival, and was fully reversed by complex colonization. Additionally, SI eosinophils of GF mice exhibited more cytoplasmic protrusions and less granule content than SPF controls. Lastly, we generated a novel strain of eosinophil-deficient GF mice. These mice displayed intestinal fibrosis and were less prone to allergic sensitization as compared to GF controls. Overall, our study demonstrates that commensal microbes regulate intestinal eosinophil frequency and function, which impacts tissue repair and allergic sensitization to food antigens. These data support a critical interplay between the commensal microbiota and intestinal eosinophils in shaping homeostatic, innate, and adaptive immune processes in health and disease.
Keywords: IgE; allergy; eosinophils; germfree; microbiota; small intestine; tissue remodeling.
Copyright © 2020 Jiménez-Saiz, Anipindi, Galipeau, Ellenbogen, Chaudhary, Koenig, Gordon, Walker, Mandur, Abed, Humbles, Chu, Erjefält, Ask, Verdú and Jordana.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

