NK Cell-Based Immune Checkpoint Inhibition
- PMID: 32117298
- PMCID: PMC7031489
- DOI: 10.3389/fimmu.2020.00167
NK Cell-Based Immune Checkpoint Inhibition
Abstract
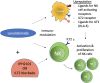
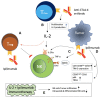
Immunotherapy, with an increasing number of therapeutic dimensions, is becoming an important mode of treatment for cancer patients. The inhibition of immune checkpoints, which are the source of immune escape for various cancers, is one such immunotherapeutic dimension. It has mainly been aimed at T cells in the past, but NK cells are a newly emerging target. Simultaneously, the number of checkpoints identified has been increasing in recent times. In addition to the classical NK cell receptors KIRs, LIRs, and NKG2A, several other immune checkpoints have also been shown to cause dysfunction of NK cells in various cancers and chronic infections. These checkpoints include the revolutionized CTLA-4, PD-1, and recently identified B7-H3, as well as LAG-3, TIGIT & CD96, TIM-3, and the most recently acknowledged checkpoint-members of the Siglecs family (Siglec-7/9), CD200 and CD47. An interesting dimension of immune checkpoints is their candidacy for dual-checkpoint inhibition, resulting in therapeutic synergism. Furthermore, the combination of immune checkpoint inhibition with other NK cell cytotoxicity restoration strategies could also strengthen its efficacy as an antitumor therapy. Here, we have undertaken a comprehensive review of the literature to date regarding NK cell-based immune checkpoints.
Keywords: cancer immunotherapy (CI); immune checkpoint; immune checkpoint inhibitors (ICI); immune therapeutics; natural killer cell (NK).
Copyright © 2020 Khan, Arooj and Wang.
Figures


References
-
- Greenberg AH. The origins of the NK cell, or a Canadian in King Ivan's court. Clin Invest Med Med Clin Exp. (1994) 17:626–31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

