Key Aspects of the Immunobiology of Haploidentical Hematopoietic Cell Transplantation
- PMID: 32117310
- PMCID: PMC7033970
- DOI: 10.3389/fimmu.2020.00191
Key Aspects of the Immunobiology of Haploidentical Hematopoietic Cell Transplantation
Abstract
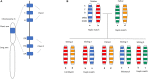
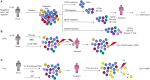
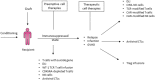
Hematopoietic stem cell transplantation from a haploidentical donor is increasingly used and has become a standard donor option for patients lacking an appropriately matched sibling or unrelated donor. Historically, prohibitive immunological barriers resulting from the high degree of HLA-mismatch included graft-vs.-host disease (GVHD) and graft failure. These were overcome with increasingly sophisticated strategies to manipulate the sensitive balance between donor and recipient immune cells. Three different approaches are currently in clinical use: (a) ex vivo T-cell depletion resulting in grafts with defined immune cell content (b) extensive immunosuppression with a T-cell replete graft consisting of G-CSF primed bone marrow and PBSC (GIAC) (c) T-cell replete grafts with post-transplant cyclophosphamide (PTCy). Intriguing studies have recently elucidated the immunologic mechanisms by which PTCy prevents GVHD. Each approach uniquely affects post-transplant immune reconstitution which is critical for the control of post-transplant infections and relapse. NK-cells play a key role in haplo-HCT since they do not mediate GVHD but can successfully mediate a graft-vs.-leukemia effect. This effect is in part regulated by KIR receptors that inhibit NK cell cytotoxic function when binding to the appropriate HLA-class I ligands. In the context of an HLA-class I mismatch in haplo-HCT, lack of inhibition can contribute to NK-cell alloreactivity leading to enhanced anti-leukemic effect. Emerging work reveals immune evasion phenomena such as copy-neutral loss of heterozygosity of the incompatible HLA alleles as one of the major mechanisms of relapse. Relapse and infectious complications remain the leading causes impacting overall survival and are central to scientific advances seeking to improve haplo-HCT. Given that haploidentical donors can typically be readily approached to collect additional stem- or immune cells for the recipient, haplo-HCT represents a unique platform for cell- and immune-based therapies aimed at further reducing relapse and infections. The rapid advancements in our understanding of the immunobiology of haplo-HCT are therefore poised to lead to iterative innovations resulting in further improvement of outcomes with this compelling transplant modality.
Keywords: NK-cells; graft-vs.-leukemia; haploidentical; immunobiology; stem cell transplantation.
Copyright © 2020 Baumeister, Rambaldi, Shapiro and Romee.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

