Spinal Muscular Atrophy in the Black South African Population: A Matter of Rearrangement?
- PMID: 32117462
- PMCID: PMC7033609
- DOI: 10.3389/fgene.2020.00054
Spinal Muscular Atrophy in the Black South African Population: A Matter of Rearrangement?
Abstract
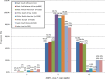
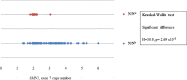
Spinal muscular atrophy (SMA) is a neuromuscular disorder, characterized by muscle atrophy and impaired mobility. A homozygous deletion of survival motor neuron 1 (SMN1), exon 7 is the main cause of SMA in ~94% of patients worldwide, but only accounts for 51% of South African (SA) black patients. SMN1 and its highly homologous centromeric copy, survival motor neuron 2 (SMN2), are located in a complex duplicated region. Unusual copy number variations (CNVs) have been reported in black patients, suggesting the presence of complex pathogenic rearrangements. The aim of this study was to further investigate the genetic cause of SMA in the black SA population. Multiplex ligation-dependent probe amplification (MLPA) testing was performed on 197 unrelated black patients referred for SMA testing (75 with a homozygous deletion of SMN1, exon 7; 50 with a homozygous deletion of SMN2, exon 7; and 72 clinically suggestive patients with no homozygous deletions). Furthermore, 122 black negative controls were tested. For comparison, 68 white individuals (30 with a homozygous deletion of SMN1, exon 7; 8 with a homozygous deletion of SMN2, exon 7 and 30 negative controls) were tested. Multiple copies (>2) of SMN1, exon 7 were observed in 50.8% (62/122) of black negative controls which could mask heterozygous SMN1 deletions and potential pathogenic CNVs. MLPA is not a reliable technique for detecting carriers in the black SA population. Large deletions extending into the rest of SMN1 and neighboring genes were more frequently observed in black patients with homozygous SMN1, exon 7 deletions when compared to white patients. Homozygous SMN2, exon 7 deletions were commonly observed in black individuals. No clear pathogenic CNVs were identified in black patients but discordant copy numbers of exons suggest complex rearrangements, which may potentially interrupt the SMN1 gene. Only 8.3% (6/72) of clinically suggestive patients had heterozygous deletions of SMN1, exon 7 (1:0) which is lower than previous SA reports of 69.5%. This study emphasizes the lack of understanding of the architecture of the SMN region as well as the cause of SMA in the black SA population. These factors need to be taken into account when counseling and performing diagnostic testing in black populations.
Keywords: South Africa; copy number variations; multiplex ligation-dependent probe amplification; rearrangement; spinal muscular atrophy; survival motor neuron 1; survival motor neuron 2.
Copyright © 2020 Vorster, Essop, Rodda and Krause.
Figures

References
-
- Barker K. (2004). At the Bench: A Laboratory Navigator, Updated Edition. 2nd edition (Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; ).
LinkOut - more resources
Full Text Sources

