Clinical Study of Nanofibrillar Cellulose Hydrogel Dressing for Skin Graft Donor Site Treatment
- PMID: 32117583
- PMCID: PMC7047117
- DOI: 10.1089/wound.2019.0982
Clinical Study of Nanofibrillar Cellulose Hydrogel Dressing for Skin Graft Donor Site Treatment
Abstract
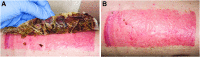
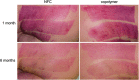
Objective: Skin graft donor site management is a concern particularly for elderly patients and patients with poor wound healing competence, and also because donor sites are a source of pain and discomfort. Although different types of dressings exist, there is no consensus regarding optimal dressing type on donor site care to promote healing, reduce pain, and improve patients' comfort. Approach: This prospective, single-center clinical trial evaluated the performance of nanofibrillar cellulose (NFC) wound dressing (FibDex® by UPM-Kymmene Corporation) for treatment of donor sites compared with a polylactide-based copolymer dressing. The study enrolled 24 patients requiring skin grafting with mean age of 49 ± 18. The primary outcome measure was wound healing time. Secondary outcomes, the epithelialization, subjective pain, the scar appearance assessed using the Patient and Observer Scar Assessment Scale (POSAS), and skin elasticity and transepidermal water loss (TEWL), were evaluated at 1 and 6 months postoperatively. Results: No statistically significant differences were observed between NFC and copolymer dressings regarding wound healing time, epithelialization, experience of pain, or TEWL. Significant differences were observed in the POSAS results for thickness and vascularity in the Observer score, in the favor of NFC over copolymer dressing. Moreover, skin elasticity was significantly improved with NFC dressing in terms of viscoelasticity and elastic modulus at 1 month postoperatively. Innovation: NFC dressing is a new, green sustainable product for wound treatment without animal or human-origin components. Conclusion: NFC dressing provides efficient wound healing at skin graft donor sites and is comparable or even preferable compared with the copolymer dressing.
Keywords: clinical study; nanofibrillar cellulose; patient; skin graft donor site treatment; wound dressing.
© Raili Koivuniemi, et al. 2019; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
K.L. and M.K. represent UPM-Kymmene Corporation, which is the manufacturer of NFC dressing. Other authors do not have potential conflicts of interest associated with this publication. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
Figures




References
-
- Uygur F, Evinc R, Ulkur E, Celikoz B. Use of lyophilized bovine collagen for split-thickness skin graft donor site management. Burns 2008;34:1011–1014 - PubMed
-
- Kolakovic R, Peltonen L, Laukkanen A, Hirvonen J, Laaksonen T. Nanofibrillar cellulose films for controlled drug delivery. Eur J Pharm Biopharm 2012;82:308–315 - PubMed
-
- Valo H, Arola S, Laaksonen P, et al. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur J Pharm Sci 2013;50:69–77 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

