Broad similarities in shoulder muscle architecture and organization across two amniotes: implications for reconstructing non-mammalian synapsids
- PMID: 32117627
- PMCID: PMC7034385
- DOI: 10.7717/peerj.8556
Broad similarities in shoulder muscle architecture and organization across two amniotes: implications for reconstructing non-mammalian synapsids
Abstract
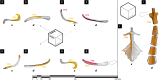
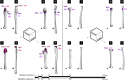
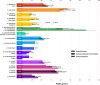
The evolution of upright limb posture in mammals may have enabled modifications of the forelimb for diverse locomotor ecologies. A rich fossil record of non-mammalian synapsids holds the key to unraveling the transition from "sprawling" to "erect" limb function in the precursors to mammals, but a detailed understanding of muscle functional anatomy is a necessary prerequisite to reconstructing postural evolution in fossils. Here we characterize the gross morphology and internal architecture of muscles crossing the shoulder joint in two morphologically-conservative extant amniotes that form a phylogenetic and morpho-functional bracket for non-mammalian synapsids: the Argentine black and white tegu Salvator merianae and the Virginia opossum Didelphis virginiana. By combining traditional physical dissection of cadavers with nondestructive three-dimensional digital dissection, we find striking similarities in muscle organization and architectural parameters. Despite the wide phylogenetic gap between our study species, distal muscle attachments are notably similar, while differences in proximal muscle attachments are driven by modifications to the skeletal anatomy of the pectoral girdle that are well-documented in transitional synapsid fossils. Further, correlates for force production, physiological cross-sectional area (PCSA), muscle gearing (pennation), and working range (fascicle length) are statistically indistinguishable for an unexpected number of muscles. Functional tradeoffs between force production and working range reveal muscle specializations that may facilitate increased girdle mobility, weight support, and active stabilization of the shoulder in the opossum-a possible signal of postural transformation. Together, these results create a foundation for reconstructing the musculoskeletal anatomy of the non-mammalian synapsid pectoral girdle with greater confidence, as we demonstrate by inferring shoulder muscle PCSAs in the fossil non-mammalian cynodont Massetognathus pascuali.
Keywords: Forelimb; Locomotion; Mammals; Musculoskeletal function; Non-mammalian synapsid; Posture; Shoulder.
© 2020 Fahn-Lai et al.
Conflict of interest statement
Stephanie E. Pierce is an Academic Editor for PeerJ.
Figures






References
-
- Alexander RM, Jayes AS, Maloiy GMO, Wathuta EM. Allometry of the leg muscles of mammals. Journal of Zoology. 1981;194(4):539–552. doi: 10.1111/j.1469-7998.1981.tb04600.x. - DOI
-
- Allen V, Molnar J, Parker W, Pollard A, Nolan G, Hutchinson JR. Comparative architectural properties of limb muscles in Crocodylidae and Alligatoridae and their relevance to divergent use of asymmetrical gaits in extant Crocodylia. Journal of Anatomy. 2014;225(6):569–582. doi: 10.1111/joa.12245. - DOI - PMC - PubMed
-
- Ashley-Ross M. Metamorphic and speed effects on Hindlimb Kinematics during Terrestrial Locomotion in the salamander Dicamptodon Tenebrosus. Journal of Experimental Biology. 1994;193:285–305. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

