Mycophenolate-induced Colitis: A Case Report with Focused Review of Literature
- PMID: 32117661
- PMCID: PMC7041651
- DOI: 10.7759/cureus.6774
Mycophenolate-induced Colitis: A Case Report with Focused Review of Literature
Abstract
Mycophenolate mofetil (MMF) is an immunosuppressive medication used for the management of various autoimmune diseases, and patients with bone marrow and solid organ transplants. Gastrointestinal side effects are seen 45% of the time and they include nausea (29%), vomiting (23%), constipation (38%), diarrhea (50%-92%), and colitis (9%). In 98% of cases, resolution of diarrhea occurs within 20 days upon discontinuation of the MMF. Data is scarce regarding approach in the treatment of MMF-induced colitis. We report a case of MMF-induced colitis diagnosed by colonoscopy and histopathology. This case illustrates the challenges encountered while managing MMF-induced colitis.
Keywords: colitis; crypt cell apoptosis; drug induced colitis; inflammatory bowel disease (ibd); mycophenolate induced colitis; ulcerative colitis.
Copyright © 2020, Farooqi et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
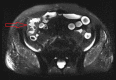

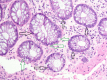

References
-
- Mycophenolate mofetil and its mechanisms of action. Allison AC, Eugui EM. Immunopharmacology. 2000;47:85–118. - PubMed
-
- Mycophenolate mofetil (MMF)-induced colitis. Dhakal P, Gami R, Giri S, Bhatt VR. Blood. 2016;128:4795.
-
- Adverse gastrointestinal effects of mycophenolate mofetil. Behrend M. https://link.springer.com/article/10.2165/00002018-200124090-00002 Drug. 2001;24:645–663. - PubMed
-
- Mycophenolate-induced colitis. Farooqi R, Kamal A, Thind K, Burke C. https://journals.lww.com/ajg/Fulltext/2018/10001/Mycophenolate_Induced_C... Am J Gastroenterol. 2018;113:0.
Publication types
LinkOut - more resources
Full Text Sources
