Update on Immunosuppression in Liver Transplantation
- PMID: 32117698
- PMCID: PMC7047305
- DOI: 10.5005/jp-journals-10018-1301
Update on Immunosuppression in Liver Transplantation
Abstract
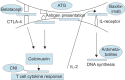
The standard therapy for decompensated end-stage chronic liver disease of any etiology and acute fulminant hepatic failure is liver transplantation (LT). Advances in immunosuppressive therapy decreased the rates of acute and chronic rejections. Thus, graft and patient survivals have significantly improved. However, long-term adverse effects of prolonged use of immunosuppressive agents such as malignancies, opportunistic infections, metabolic disorders, and other organ toxicities have now become a major concern. Consequently, alternative approaches are needed to deescalate the customary drugs and their side effects. Therapy must be individualized and additional preventive measures should be taken by patients with particular risk factors or predisposed to certain adverse effects. Current opinion favors a combination of agents with different mechanism of actions and toxicity profiles. Corticosteroids are employed in immediate and early postoperative period. Although they have a pronounced side effect profile, calcineurin inhibitors (CNIs) are still the backbone of early and late phase immunosuppressive regimens because of their proved efficacy. Antimetabolites are frequent choices for steroid and/or CNI-sparing strategies. Studies also have established a role for mammalian target of rapamycin (mTOR) inhibitors in specific groups of recipients. Biologic agents are a hot topic of interest and made their way into current strategies for induction. Agents extrapolated from other transplantation or immunologic experience are being evaluated.
How to cite this article: Tasdogan BE, Ma M, Simsek C, et al. Update on Immunosuppression in Liver Transplantation. Euroasian J Hepato-Gastroenterol 2019;9(2):96-101.
Keywords: Adverse effects; Immunosuppression; Liver transplantation.
Copyright © 2019; Jaypee Brothers Medical Publishers (P) Ltd.
Conflict of interest statement
Source of support: Nil Conflict of interest: None
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
