High Expression of Integrin α3 Predicts Poor Prognosis and Promotes Tumor Metastasis and Angiogenesis by Activating the c-Src/Extracellular Signal-Regulated Protein Kinase/Focal Adhesion Kinase Signaling Pathway in Cervical Cancer
- PMID: 32117712
- PMCID: PMC7033469
- DOI: 10.3389/fonc.2020.00036
High Expression of Integrin α3 Predicts Poor Prognosis and Promotes Tumor Metastasis and Angiogenesis by Activating the c-Src/Extracellular Signal-Regulated Protein Kinase/Focal Adhesion Kinase Signaling Pathway in Cervical Cancer
Abstract
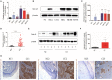
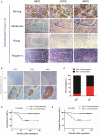
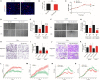
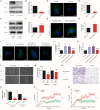
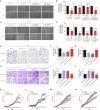
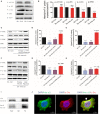
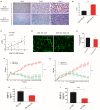
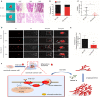
Background: Cervical cancer remains a leading cause of death in women due to metastasis to distant tissues and organs. Integrins are involved in cancer metastasis. However, whether integrin α3 participates in cervical cancer metastasis is under investigation. In this study, we explored the effect and detailed mechanism through which integrin α3 regulates cervical cell migration, invasion, and angiogenesis. Methods: First, we explored the mRNA and protein expression levels of integrin α3 in cervical cancer cell lines and tissue samples obtained from patients. After knocking down the expression of integrin α3 using shRNA, the proliferation, migration, and invasion of cervical cancer cells, as well as the possible signaling pathways involved, were investigated in vitro. In addition, tube formation, proliferation, and migration of human umbilical vein endothelial cells were tested to identify their effect on angiogenesis. Zebrafish tumor migration and nude mouse lung metastasis models were utilized for the in vivo analysis. Results: We examined samples from 142 patients with cervical cancer and 20 normal cervixes. Integrin α3 was highly expressed in patients and predicted poor overall survival and disease-free survival. In SiHa cells, treatment with integrin α3 shRNA induced the phosphorylation of protein focal adhesion kinase and enhanced focal adhesion. These events were mediated by the activation of c-Src and extracellular signal-regulated protein kinase cascades. Consequently, integrin α3 increased the migratory ability of SiHa cells. In addition, knockdown of integrin α3 decreased the tube formation, proliferation, and migration of human umbilical vein endothelial cells, as well as the levels of matrix metalloproteinase-9, indicating its effect on angiogenesis. Stable transfection with integrin α3 shRNA reduced the migratory ability of SiHa cells in the zebrafish model and diminished lung metastasis in the xenograft mouse model. Conclusion: Integrin α3 recruits the c-Src/extracellular signal-regulated protein kinase cascade, leading to phosphorylation of focal adhesion kinase. Moreover, it regulates focal adhesion, endowing cervical cancer cells with potentiated migratory and invasive ability, and promotes angiogenesis via matrix metalloproteinase-9. Our findings may shed light on the mechanism involved in cervical cancer metastasis and highlight integrin α3 as a candidate prognostic biomarker and therapeutic target in patients with cervical cancer.
Keywords: angiogenesis; cervical cancer; focal adhesion kinase; integrin α3; metastasis.
Copyright © 2020 Du, Wang, Liu, Shang, Huang, Liao, Qin, Chen, Liu, Liu and Yao.
Figures
Similar articles
-
Src activity alters alpha3 integrin expression in colon tumor cells.Clin Exp Metastasis. 2009;26(2):77-87. doi: 10.1007/s10585-008-9215-x. Epub 2008 Oct 7. Clin Exp Metastasis. 2009. PMID: 18839319
-
Vascular endothelial growth factor C enhances cervical cancer migration and invasion via activation of focal adhesion kinase.Gynecol Endocrinol. 2013 Jan;29(1):20-4. doi: 10.3109/09513590.2012.705387. Epub 2012 Jul 21. Gynecol Endocrinol. 2013. PMID: 22817694
-
TGF-beta1 (transforming growth factor-beta1)-mediated adhesion of gastric carcinoma cells involves a decrease in Ras/ERKs (extracellular-signal-regulated kinases) cascade activity dependent on c-Src activity.Biochem J. 2004 Apr 1;379(Pt 1):141-50. doi: 10.1042/BJ20031408. Biochem J. 2004. PMID: 14720123 Free PMC article.
-
Focal adhesion kinase and its potential involvement in tumor invasion and metastasis.Head Neck. 1998 Dec;20(8):745-52. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. Head Neck. 1998. PMID: 9790298 Review.
-
The interplay between Src and integrins in normal and tumor biology.Oncogene. 2004 Oct 18;23(48):7928-46. doi: 10.1038/sj.onc.1208080. Oncogene. 2004. PMID: 15489911 Review.
Cited by
-
Integrins Can Act as Suppressors of Ras-Mediated Oncogenesis in the Drosophila Wing Disc Epithelium.Cancers (Basel). 2023 Nov 15;15(22):5432. doi: 10.3390/cancers15225432. Cancers (Basel). 2023. PMID: 38001693 Free PMC article.
-
FASN promotes lymph node metastasis in cervical cancer via cholesterol reprogramming and lymphangiogenesis.Cell Death Dis. 2022 May 21;13(5):488. doi: 10.1038/s41419-022-04926-2. Cell Death Dis. 2022. PMID: 35597782 Free PMC article.
-
ITGA3 promotes pancreatic cancer progression through HIF1α- and c-Myc-driven glycolysis in a collagen I-dependent autocrine manner.Cancer Gene Ther. 2025 Feb;32(2):240-253. doi: 10.1038/s41417-024-00864-7. Epub 2024 Dec 17. Cancer Gene Ther. 2025. PMID: 39690180
-
RNA-seq reveals the diverse effects of substrate stiffness on epidermal ovarian cancer cells.Aging (Albany NY). 2020 Oct 22;12(20):20493-20511. doi: 10.18632/aging.103906. Epub 2020 Oct 22. Aging (Albany NY). 2020. PMID: 33091877 Free PMC article.
-
Construction of a prognostic risk assessment model for lung adenocarcinoma based on Integrin β family-related genes.J Clin Lab Anal. 2022 Jun;36(6):e24419. doi: 10.1002/jcla.24419. Epub 2022 Apr 11. J Clin Lab Anal. 2022. PMID: 35403268 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

