Caveolin-1 Promotes Chemoresistance of Gastric Cancer Cells to Cisplatin by Activating WNT/β-Catenin Pathway
- PMID: 32117718
- PMCID: PMC7008851
- DOI: 10.3389/fonc.2020.00046
Caveolin-1 Promotes Chemoresistance of Gastric Cancer Cells to Cisplatin by Activating WNT/β-Catenin Pathway
Abstract
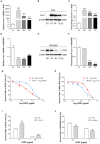
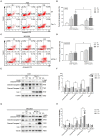
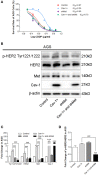
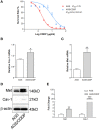
Drug resistance is a major challenge for chemotherapy in treating human gastric cancer (GC), as the underlying molecular mechanism of chemoresistance in GC remains unknown. Caveolin-1 (Cav-1) is a scaffold protein of plasma membrane caveolae that acts as a tumor modulator by interacting with several cell signals. In this research, we showed that the survival rate of GC cells to cisplatin (CDDP) increased in the presence of Cav-1. Moreover, Cav-1 overexpression inhibited cisplatin-induced apoptosis and improved the survival rate of GC cells. Cav-1 overexpression and knock-down experiments indicated that Cav-1 expression stimulated wingless-type MMTV integration site (WNTs) pathway through the phosphorylation of LRP6 and dephosphorylation of β-catenin. Cav-1 was positively associated with the increase of WNT downstream target gene Met, which led to the activation of HER2 signaling. Moreover, our results demonstrated that the expression of Cav-1 and Met were positively associated with the resistance of GC cells to cisplatin. Collectively, Cav-1 enhances the cisplatin-resistance of GC cells by activating the WNT signaling pathway and Met-HER2 crosstalk. Understanding the role of Cav-1 in the chemoresistance of GC would help to develop novel therapies for a better treatment outcome of GC patients.
Keywords: Caveolin-1; WNT/β-catenin pathway; chemoresistance; cisplatin; gastric cancer.
Copyright © 2020 Wang, Lu, Dai, Fu, Hao, Zhao, Chen and Fu.
Figures



Similar articles
-
WNT6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells.Oncogene. 2013 Jan 17;32(3):375-87. doi: 10.1038/onc.2012.40. Epub 2012 Feb 27. Oncogene. 2013. PMID: 22370641
-
Caveolin-1 mediates chemoresistance in cisplatin-resistant ovarian cancer cells by targeting apoptosis through the Notch-1/Akt/NF-κB pathway.Oncol Rep. 2015 Dec;34(6):3256-63. doi: 10.3892/or.2015.4320. Oncol Rep. 2015. PMID: 26503358
-
Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a.Int J Biol Macromol. 2018 Feb;107(Pt B):2620-2629. doi: 10.1016/j.ijbiomac.2017.10.154. Epub 2017 Oct 26. Int J Biol Macromol. 2018. PMID: 29080815
-
New glimpses of caveolin-1 functions in embryonic development and human diseases.Front Biol (Beijing). 2011;6(5):367. doi: 10.1007/s11515-011-1132-8. Epub 2011 Oct 1. Front Biol (Beijing). 2011. PMID: 32215005 Free PMC article. Review.
-
Multifaceted Roles of Caveolin-1 in Lung Cancer: A New Investigation Focused on Tumor Occurrence, Development and Therapy.Cancers (Basel). 2020 Jan 25;12(2):291. doi: 10.3390/cancers12020291. Cancers (Basel). 2020. PMID: 31991790 Free PMC article. Review.
Cited by
-
Magnesium-Assisted Cisplatin Inhibits Bladder Cancer Cell Survival by Modulating Wnt/β-Catenin Signaling Pathway.Front Pharmacol. 2022 Jan 27;12:804615. doi: 10.3389/fphar.2021.804615. eCollection 2021. Front Pharmacol. 2022. PMID: 35153759 Free PMC article.
-
Signaling pathways and therapeutic interventions in gastric cancer.Signal Transduct Target Ther. 2022 Oct 8;7(1):358. doi: 10.1038/s41392-022-01190-w. Signal Transduct Target Ther. 2022. PMID: 36209270 Free PMC article. Review.
-
β-catenin as a key regulator of the cisplatin response in tumor cells.Clin Exp Med. 2025 Jun 15;25(1):206. doi: 10.1007/s10238-025-01757-1. Clin Exp Med. 2025. PMID: 40517336 Free PMC article. Review.
-
The role of caveolin-1 in the biofate and efficacy of anti-tumor drugs and their nano-drug delivery systems.Acta Pharm Sin B. 2021 Apr;11(4):961-977. doi: 10.1016/j.apsb.2020.11.020. Epub 2020 Nov 28. Acta Pharm Sin B. 2021. PMID: 33996409 Free PMC article. Review.
-
CAV1 alleviated CaOx stones formation via suppressing autophagy-dependent ferroptosis.PeerJ. 2022 Sep 15;10:e14033. doi: 10.7717/peerj.14033. eCollection 2022. PeerJ. 2022. PMID: 36128191 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

