Amplification of Replication Competent HIV-1 by Adoptive Transfer of Human Cells From Infected Humanized Mice
- PMID: 32117811
- PMCID: PMC7026001
- DOI: 10.3389/fcimb.2020.00038
Amplification of Replication Competent HIV-1 by Adoptive Transfer of Human Cells From Infected Humanized Mice
Abstract
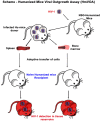
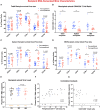
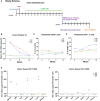
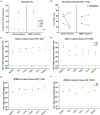
Detection of latent human immunodeficiency virus type 1 (HIV-1) in "putative" infectious reservoirs is required for determining treatment efficiency and for viral elimination strategies. Such tests require induction of replication competent provirus and quantitative testing of viral load for validation. Recently, humanized mice were employed in the development of such tests by employing a murine viral outgrowth assay (mVOA). Here blood cells were recovered from virus infected antiretroviral therapy suppressed patients. These cells were adoptively transferred to uninfected humanized mice where replication competent virus was recovered. Prior reports supported the notion that an mVOA assay provides greater sensitivity than cell culture-based quantitative VOA tests for detection of latent virus. In the current study, the mVOA assays was adapted using donor human hematopoietic stem cells-reconstituted mice to affirm research into HIV-1 elimination. We simulated an antiretroviral therapy (ART)-treated virus-infected human by maintaining the infected humanized mice under suppressive treatment. This was operative prior to human cell adoptive transfers. Replication-competent HIV-1 was easily detected in recipient animals from donors with undetectable virus in plasma. Moreover, when the assay was used to investigate viral presence in tissue reservoirs, quantitative endpoints were determined in "putative" viral reservoirs not possible in human sample analyses. We conclude that adoptive transfer of cells between humanized mice is a sensitive and specific assay system for detection of replication competent latent HIV-1.
Keywords: HIV-1 infection; adoptive transfers; humanized mice; lymphoid tissue; viral latency; viral outgrowth assays; viral reservoirs.
Copyright © 2020 Su, Sravanam, Gorantla, Kaminski, Khalili, Poluektova, Gendelman and Dash.
Figures

Similar articles
-
Recovery of Latent HIV-1 from Brain Tissue by Adoptive Cell Transfer in Virally Suppressed Humanized Mice.J Neuroimmune Pharmacol. 2021 Dec;16(4):796-805. doi: 10.1007/s11481-021-10011-w. Epub 2021 Sep 15. J Neuroimmune Pharmacol. 2021. PMID: 34528173 Free PMC article.
-
Ultra-Sensitive HIV-1 Latency Viral Outgrowth Assays Using Humanized Mice.Front Immunol. 2018 Mar 5;9:344. doi: 10.3389/fimmu.2018.00344. eCollection 2018. Front Immunol. 2018. PMID: 29556230 Free PMC article. Review.
-
A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads.J Infect Dis. 2015 Nov 1;212(9):1387-96. doi: 10.1093/infdis/jiv230. Epub 2015 Apr 15. J Infect Dis. 2015. PMID: 25883388 Free PMC article.
-
Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir.mBio. 2017 Aug 15;8(4):e01186-17. doi: 10.1128/mBio.01186-17. mBio. 2017. PMID: 28811349 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
Cited by
-
HIV-1 cure strategies: why CRISPR?Expert Opin Biol Ther. 2021 Jun;21(6):781-793. doi: 10.1080/14712598.2021.1865302. Epub 2021 Feb 7. Expert Opin Biol Ther. 2021. PMID: 33331178 Free PMC article. Review.
-
CRISPR editing of CCR5 and HIV-1 facilitates viral elimination in antiretroviral drug-suppressed virus-infected humanized mice.Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2217887120. doi: 10.1073/pnas.2217887120. Epub 2023 May 1. Proc Natl Acad Sci U S A. 2023. PMID: 37126704 Free PMC article.
-
Humanized Mice for Infectious and Neurodegenerative disorders.Retrovirology. 2021 Jun 5;18(1):13. doi: 10.1186/s12977-021-00557-1. Retrovirology. 2021. PMID: 34090462 Free PMC article. Review.
-
Single-cell profiling reveals a conserved role for hypoxia-inducible factor signaling during human craniotomy infection.Cell Rep Med. 2024 Nov 19;5(11):101790. doi: 10.1016/j.xcrm.2024.101790. Epub 2024 Oct 18. Cell Rep Med. 2024. PMID: 39426374 Free PMC article.
-
Off-Target Analysis in Gene Editing and Applications for Clinical Translation of CRISPR/Cas9 in HIV-1 Therapy.Front Genome Ed. 2021 Aug 17;3:673022. doi: 10.3389/fgeed.2021.673022. eCollection 2021. Front Genome Ed. 2021. PMID: 34713260 Free PMC article. Review.
References
-
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., et al. . (1986). Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59, 284–291. 10.1128/JVI.59.2.284-291.1986 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

