Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence
- PMID: 32117816
- PMCID: PMC7031155
- DOI: 10.3389/fcimb.2020.00049
Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence
Abstract
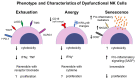
There is a growing body of literature demonstrating the importance of T cell exhaustion in regulating and shaping immune responses to pathogens and cancer. Simultaneously, the parallel development of therapeutic antibodies targeting inhibitory molecules associated with immune exhaustion (such as PD-1, but also TIGIT, and LAG-3) has led to a revolution in oncology with dramatic benefits in a growing list of solid and hematologic malignancies. Given this success in reinvigorating exhausted T cells and the related anti-tumor effects, there are increasing efforts to apply immune checkpoint blockade to other exhausted immune cells beyond T cells. One approach involves the reinvigoration of "exhausted" NK cells, a non-T, non-B lymphoid cell of the innate immune system. However, in contrast to the more well-defined and established molecular, genetic, and immunophenotypic characteristics of T cell exhaustion, a consensus on the defining functional and phenotypic features of NK "exhaustion" is less clear. As is well-known from T cell biology, separate and distinct molecular and cellular processes including senescence, anergy and exhaustion can lead to diminished immune effector function with different implications for immune regulation and recovery. For NK cells, it is unclear if exhaustion, anergy, and senescence entail separate and distinct entities of dysfunction, though all are typically characterized by decreased effector function or proliferation. In this review, we seek to define these distinct spheres of NK cell dysfunction, analyzing how they have been shown to impact NK biology and clinical applications, and ultimately highlight key characteristics in NK cell function, particularly in relation to the role of "exhaustion."
Keywords: NK cells; NK dysfunction; NK exhaustion; immune dysfunction; natural killer cells.
Copyright © 2020 Judge, Murphy and Canter.
Figures
Similar articles
-
Unraveling exhaustion in adaptive and conventional NK cells.J Leukoc Biol. 2020 Oct;108(4):1361-1368. doi: 10.1002/JLB.4MR0620-091R. Epub 2020 Jul 29. J Leukoc Biol. 2020. PMID: 32726880 Free PMC article. Review.
-
Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity.Nat Immunol. 2018 Jul;19(7):723-732. doi: 10.1038/s41590-018-0132-0. Epub 2018 Jun 18. Nat Immunol. 2018. PMID: 29915296
-
Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy.Front Immunol. 2020 Jun 23;11:1295. doi: 10.3389/fimmu.2020.01295. eCollection 2020. Front Immunol. 2020. PMID: 32714324 Free PMC article. Review.
-
The Rise of NK Cell Checkpoints as Promising Therapeutic Targets in Cancer Immunotherapy.Front Immunol. 2019 Oct 17;10:2354. doi: 10.3389/fimmu.2019.02354. eCollection 2019. Front Immunol. 2019. PMID: 31681269 Free PMC article. Review.
-
Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells.Front Immunol. 2018 Sep 10;9:2041. doi: 10.3389/fimmu.2018.02041. eCollection 2018. Front Immunol. 2018. PMID: 30250471 Free PMC article. Review.
Cited by
-
Impact of immunosenescence and inflammaging on the effects of immune checkpoint inhibitors.Cancer Pathog Ther. 2023 Aug 9;2(1):24-30. doi: 10.1016/j.cpt.2023.08.001. eCollection 2024 Jan. Cancer Pathog Ther. 2023. PMID: 38328711 Free PMC article. Review.
-
Outsmarting trogocytosis to boost CAR NK/T cell therapy.Mol Cancer. 2023 Nov 16;22(1):183. doi: 10.1186/s12943-023-01894-9. Mol Cancer. 2023. PMID: 37974170 Free PMC article. Review.
-
AI-guided discovery of the invariant host response to viral pandemics.EBioMedicine. 2021 Jun;68:103390. doi: 10.1016/j.ebiom.2021.103390. Epub 2021 Jun 11. EBioMedicine. 2021. PMID: 34127431 Free PMC article.
-
Engineering the next generation of CAR-NK immunotherapies.Int J Hematol. 2021 Nov;114(5):554-571. doi: 10.1007/s12185-021-03209-4. Epub 2021 Aug 28. Int J Hematol. 2021. PMID: 34453686 Free PMC article. Review.
-
Engineered human pluripotent stem cell-derived natural killer cells with PD-L1 responsive immunological memory for enhanced immunotherapeutic efficacy.Bioact Mater. 2023 Apr 5;27:168-180. doi: 10.1016/j.bioactmat.2023.03.018. eCollection 2023 Sep. Bioact Mater. 2023. PMID: 37091063 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

