Characteristics Associated With Recruitment and Re-contact in Mayo Clinic Biobank
- PMID: 32117849
- PMCID: PMC7010638
- DOI: 10.3389/fpubh.2020.00009
Characteristics Associated With Recruitment and Re-contact in Mayo Clinic Biobank
Abstract
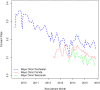
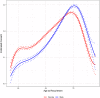
Objective: To better understand the characteristics associated with a participant's willingness to consent to the Mayo Clinic Biobank (MCB) and examine factors associated with willingness to participate in follow-up studies embedded within MCB that require re-contact and participant approval. Participants and Methods: Consent rates were compared across patient demographics to the MCB. Rates of participation to follow-up studies were also compared across demographics and request types. Results: Among 272,102 Mayo Clinic patients invited to the MCB, 48,314 (19%) consented across the three recruitment sites within 90 days of initial invitation. A significant age by gender interaction was identified, showing young males consent at a lower rate than young females and older males consent at a higher rate than older females. Over the recruitment time frame of 2009-2015, there was a significant decrease in consent rates (decline of 2.5%/year). Of the 57,041 consented MCB participants, 33,487 participants (59%) have been invited to participate in follow-up studies via re-contact. Follow-up studies of the MCB may require participants to provide additional samples, complete questionnaires, and/or release their identity to a research team. MCB participants have been invited to enroll in a median of two studies (IQR: 1-3). Seventy-one percent of participants consented to at least one follow-up study, with individual follow-up study consent rates ranging from 14 to 87% depending on study type, with a median consent rate of 61% (IQR: 47-70%). Studies requesting return of a questionnaire had the highest participation rates. White participants, older participants, and participants with some college or a degree were significantly more likely to participate to follow-up studies, while there was no association with gender. Conclusion: Consent rates among younger and non-white patients were lower than in older, white patients. However, we also found that participation rates among those already enrolled in the biobank were much higher than those seen in new recruitment efforts, external to an existing biobank. We thus demonstrate an important way that biobanks can advance precision medicine goals: through provision of populations from which studies can draw participants for future studies.
Keywords: biobanks; consent; follow-up study; participation; recruitment.
Copyright © 2020 Hathcock, Kirt, Ryu, Bublitz, Gupta, Wang, Thibodeau, Larson, Cicek, Cerhan and Olson.
Figures
References
-
- National Institutes of Health All of Us. (2019). Available online at: https://allofus.nih.gov/about/all-us-research-program-overview (accessed October 24, 2019).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

