Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering
- PMID: 32117879
- PMCID: PMC7028759
- DOI: 10.3389/fchem.2020.00053
Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering
Abstract
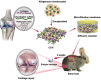
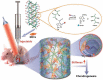
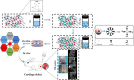
Cartilage injury originating from trauma or osteoarthritis is a common joint disease that can bring about an increasing social and economic burden in modern society. On account of its avascular, neural, and lymphatic characteristics, the poor migration ability of chondrocytes, and a low number of progenitor cells, the self-healing ability of cartilage defects has been significantly limited. Natural hydrogels, occurring abundantly with characteristics such as high water absorption, biodegradation, adjustable porosity, and biocompatibility like that of the natural extracellular matrix (ECM), have been developed into one of the most suitable scaffold biomaterials for the regeneration of cartilage in material science and tissue engineering. Notably, natural hydrogels derived from sources such as animal or human cadaver tissues possess the bionic mechanical behaviors of physiological cartilage that are required for usage as articular cartilage substitutes, by which the enhanced chondrogenic phenotype ability may be achieved by facilely embedding living cells, controlling degradation profiles, and releasing stimulatory growth factors. Hence, we summarize an overview of strategies and developments of the various kinds and functions of natural hydrogels for cartilage tissue engineering in this review. The main concepts and recent essential research found that great challenges like vascularity, clinically relevant size, and mechanical performances were still difficult to overcome because the current limitations of technologies need to be severely addressed in practical settings, particularly in unpredictable preclinical trials and during future forays into cartilage regeneration using natural hydrogel scaffolds with high mechanical properties. Therefore, the grand aim of this current review is to underpin the importance of preparation, modification, and application for the high performance of natural hydrogels for cartilage tissue engineering, which has been achieved by presenting a promising avenue in various fields and postulating real-world respective potentials.
Keywords: cartilage tissue engineering; hydrogel scaffolds; mechanical property; natural hydrogel; regenerative medicine.
Copyright © 2020 Bao, Li, Yang, Wan, Wang, Bi and Li.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

