Growing CeO2 Nanoparticles Within the Nano-Porous Architecture of the SiO2 Aerogel
- PMID: 32117882
- PMCID: PMC7018665
- DOI: 10.3389/fchem.2020.00057
Growing CeO2 Nanoparticles Within the Nano-Porous Architecture of the SiO2 Aerogel
Abstract
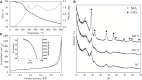
In this study, new CeO2-SiO2 aerogel nanocomposites obtained by controlled growth of CeO2 nanoparticles within the highly porous matrix of a SiO2 aerogel are presented. The nanocomposites have been synthesized via a sol-gel route, employing cerium (III) nitrate as the CeO2 precursor and selected surfactants to control the growth of the CeO2 nanoparticles, which occurs during the supercritical drying of the aerogels. Samples with different loading of the CeO2 dispersed phase, ranging from 5 to 15%, were obtained. The nanocomposites showed the morphological features typical of the SiO2 aerogels such as open mesoporosity with surface area values up to 430 m2·g-1. TEM and XRD characterizations show that nanocrystals of the dispersed CeO2 nanophase grow within the aerogel already during the supercritical drying process, with particle sizes in the range of 3 to 5 nm. TEM in particular shows that the CeO2 nanoparticles are well-distributed within the aerogel matrix. We also demonstrate the stability of the nanocomposites under high temperature conditions, performing thermal treatments in air at 450 and 900°C. Interestingly, the CeO2 nanoparticles undergo a very limited crystal growth, with sizes up to only 7 nm in the case of the sample subjected to a 900°C treatment.
Keywords: aerogel; ceria; nanocomposites; nanoporous materials; surfactants.
Copyright © 2020 Caddeo, Loche, Casula and Corrias.
Figures


References
-
- Akinc M., Sordelet D. (1987). Preparation of yttrium, lanthanum, cerium, and neodymium basic carbonate particles by homogeneous precipitation. Adv. Ceram. Mater. 2, 232–237. 10.1111/j.1551-2916.1987.tb00087.x - DOI
-
- Ameen K. B., Rajasekar K., Rajasekharan T. (2007). Silver nanoparticles in mesoporous aerogel exhibiting selective catalytic oxidation of benzene in CO2 free air. Catal. Lett. 119, 289–295. 10.1007/s10562-007-9233-3 - DOI
-
- Amonette J. E., Matyáš J. (2017). Functionalized silica aerogels for gas-phase purification, sensing, and catalysis: a review. Micropor. Mater. 250, 100–119. 10.1016/j.micromeso.2017.04.055 - DOI
-
- Barrett E. P., Joyner L. G., Halenda P. P. (1951). The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 73, 373–380. 10.1021/ja01145a126 - DOI
LinkOut - more resources
Full Text Sources

