T Cell Dysfunction and Exhaustion in Cancer
- PMID: 32117960
- PMCID: PMC7027373
- DOI: 10.3389/fcell.2020.00017
T Cell Dysfunction and Exhaustion in Cancer
Abstract
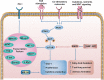
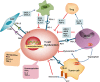
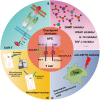
Tumor immunotherapy is a promising therapeutic strategy for patients with advanced cancers. T cells are key mediators of antitumor function that specifically recognize and react to tumor-expressing antigens and have proven critical for cancer immunotherapy. However, T cells are not as effective against cancer as expected. This is partly because T cells enter a dysfunctional or exhausted state, which is characterized by sustained expression of inhibitory receptors and a transcriptional state distinct from that of functional effector or memory T cells. T cell dysfunction induces the out of control of tumors. Recently, T cell dysfunction has been investigated in many experimental and clinical settings. The molecular definition of T cell dysfunction and the underlying causes of the T cell dysfunction has been advanced regardless of the fact that the pathways involved are not well elucidated, which proposing promising therapeutic opportunities in clinic. In this review, we will discuss the recent advances in the molecular mechanisms that affect TME and induce T cell dysfunction, and the development of promising immunotherapies to counteract the mechanisms of tumor-induced T cell dysfunction. Better understanding these underlying mechanisms may lead to new strategies to improve the clinical outcome of patients with cancer.
Keywords: T cells dysfunction; cancer immunotherapy; extrinsic factors; intrinsic factors; tumor microenvironment.
Copyright © 2020 Zhang, Liu, Zhang, Qiao, Zhang and Zhang.
Figures
References
-
- Benner B., Scarberry L., Suarez-Kelly L. P., Duggan M. C., Campbell A. R., Smith E., et al. (2019). Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J. Immunother. Cancer 7:140. 10.1186/s40425-019-0622-0 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

