The Lysosomal Membrane Protein Lamp2 Alleviates Lysosomal Cell Death by Promoting Autophagic Flux in Ischemic Cardiomyocytes
- PMID: 32117965
- PMCID: PMC7019187
- DOI: 10.3389/fcell.2020.00031
The Lysosomal Membrane Protein Lamp2 Alleviates Lysosomal Cell Death by Promoting Autophagic Flux in Ischemic Cardiomyocytes
Abstract
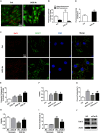
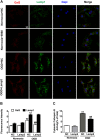
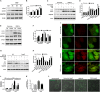
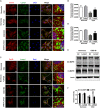
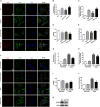
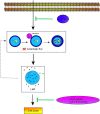
Lysosomal membrane permeabilization (LMP) has recently been recognized as an important cell death pathway in various cell types. However, studies regarding the correlation between LMP and cardiomyocyte death are scarce. Lysosomal membrane-associated protein 2 (Lamp2) is an important component of lysosomal membranes and is involved in both autophagy and LMP. In the present study, we found that the protein content of Lamp2 gradually decreased in response to oxygen, glucose and serum deprivation (OGD) treatment in vitro. To further elucidate its role in ischemic cardiomyocytes, particularly with respect to autophagy and LMP, we infected cardiomyocytes with adenovirus carrying full-length Lamp2 to restore its protein level in cells. We found that OGD treatment resulted in the occurrence of LMP and a decline in the viability of cardiomyocytes, which were remarkably reversed by Lamp2 restoration. Exogenous expression of Lamp2 also significantly alleviated the autophagic flux blockade induced by OGD treatment by promoting the trafficking of cathepsin B (Cat B) and cathepsin D (Cat D). Through drug intervention and gene regulation to alleviate and exacerbate autophagic flux blockade respectively, we found that impaired autophagic flux in response to ischemic injury contributed to the occurrence of LMP in cardiomyocytes. In conclusion, our present data suggest that Lamp2 overexpression can improve autophagic flux blockade probably by promoting the trafficking of cathepsins and consequently conferring cardiomyocyte resistance against lysosomal cell death (LCD) that is induced by ischemic injury. These results may indicate a new therapeutic target for ischemic heart damage.
Keywords: LMP; Lamp2; OGD; autophagic flux; cardiomyocytes.
Copyright © 2020 Cui, Zhao, Ye, Yang, Huang, Jiang, Zhang, Jia, Zhang and Huang.
Figures

References
-
- de Duve C. (2005). The lysosometurns fifty. Nat. Cell Biol. 7 847–849. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

