CDP-Diacylglycerol Synthases (CDS): Gateway to Phosphatidylinositol and Cardiolipin Synthesis
- PMID: 32117988
- PMCID: PMC7018664
- DOI: 10.3389/fcell.2020.00063
CDP-Diacylglycerol Synthases (CDS): Gateway to Phosphatidylinositol and Cardiolipin Synthesis
Abstract
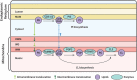
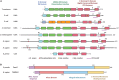
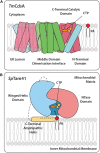
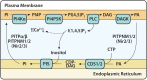
Cytidine diphosphate diacylglycerol (CDP-DAG) is a key intermediate in the synthesis of phosphatidylinositol (PI) and cardiolipin (CL). Both PI and CL have highly specialized roles in cells. PI can be phosphorylated and these phosphorylated derivatives play major roles in signal transduction, membrane traffic, and maintenance of the actin cytoskeletal network. CL is the signature lipid of mitochondria and has a plethora of functions including maintenance of cristae morphology, mitochondrial fission, and fusion and for electron transport chain super complex formation. Both lipids are synthesized in different organelles although they share the common intermediate, CDP-DAG. CDP-DAG is synthesized from phosphatidic acid (PA) and CTP by enzymes that display CDP-DAG synthase activities. Two families of enzymes, CDS and TAMM41, which bear no sequence or structural relationship, have now been identified. TAMM41 is a peripheral membrane protein localized in the inner mitochondrial membrane required for CL synthesis. CDS enzymes are ancient integral membrane proteins found in all three domains of life. In mammals, they provide CDP-DAG for PI synthesis and for phosphatidylglycerol (PG) and CL synthesis in prokaryotes. CDS enzymes are critical for maintaining phosphoinositide levels during phospholipase C (PLC) signaling. Hydrolysis of PI (4,5) bisphosphate by PLC requires the resynthesis of PI and CDS enzymes catalyze the rate-limiting step in the process. In mammals, the protein products of two CDS genes (CDS1 and CDS2) localize to the ER and it is suggested that CDS2 is the major CDS for this process. Expression of CDS enzymes are regulated by transcription factors and CDS enzymes may also contribute to CL synthesis in mitochondria. Studies of CDS enzymes in protozoa reveal spatial segregation of CDS enzymes from the rest of the machinery required for both PI and CL synthesis identifying a key gap in our understanding of how CDP-DAG can cross the different membrane compartments in protozoa and in mammals.
Keywords: CDP-diacylglycerol; TAMM41; endoplasmic reticulum; lipid synthesis; mitochondria; phosphatidic acid; phosphatidylinositol; phospholipase C.
Copyright © 2020 Blunsom and Cockcroft.
Figures
References
-
- Anderson K. E., Kielkowska A., Durrant T. N., Juvin V., Clark J., Stephens L. R., et al. (2013). Lysophosphatidylinositol-acyltransferase-1 (LPIAT1) is required to maintain physiological levels of PtdIns and PtdInsP(2) in the mouse. PLoS. One 8:e58425. 10.1371/journal.pone.0058425 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

