Study on structural atrophy changes and functional connectivity measures in Alzheimer's disease
- PMID: 32118092
- PMCID: PMC7043284
- DOI: 10.1117/1.JMI.7.1.016002
Study on structural atrophy changes and functional connectivity measures in Alzheimer's disease
Abstract
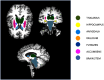
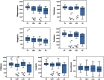
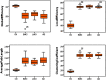
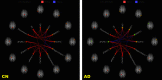
Alzheimer's disease (AD) is characterized by the progressive accumulation of neurofibrillary tangles associated with amyloid plaques. We used 80 resting-state functional magnetic resonance imaging and 80 images acquired using MP-RAGE (magnetization-prepared rapid acquisition gradient echo) from Alzheimer's Disease Neuroimaging Initiative data to detect atrophy changes and functional connectivity patterns of the default mode networks (DMNs). The study subjects were classified into four groups (each with ) based on their Mini-Mental State Examination (MMSE) score as follows: cognitively normal (CN), early mild cognitive impairment, late mild cognitive impairment, and AD. The resting-state functional connectivity of the DMN was examined between the groups using the CONN functional connectivity toolbox. Loss of gray matter in AD was observed. Atrophy measured by the volume of selected subcortical regions, using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library's Integrated Registration and Segmentation Tool (FIRST), revealed significant volume loss in AD when compared to CN ( ). DMNs were selected to assess functional connectivity. The negative connectivity of DMN increased in AD group compared to controls. Graph theory parameters, such as global and local efficiency, betweenness centrality, average path length, and cluster coefficient, were computed. Relatively higher correlation between MMSE and functional metrics ( , ) was observed as compared to atrophy measures ( , ). In addition, the receiver operating characteristic analysis showed large area under the curve ( ) for functional parameters ( ), compared to morphometric changes ( ). In summary, it is observed that the functional connectivity measures may serve a better predictor in comparison to structural atrophy changes. We postulate that functional connectivity measures have the potential to evolve as a marker for the early detection of AD.
Keywords: Alzheimer’s disease; default mode network; graph theory analysis; resting-state functional magnetic resonance imaging.
© 2020 Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

