In Vitro and Ex Vivo Efficacy of Novel Trp-Arg Rich Analogue of α-MSH against Staphylococcus aureus
- PMID: 32118141
- PMCID: PMC7045321
- DOI: 10.1021/acsomega.9b03307
In Vitro and Ex Vivo Efficacy of Novel Trp-Arg Rich Analogue of α-MSH against Staphylococcus aureus
Abstract
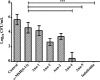
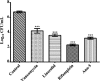
Antimicrobial peptides (AMPs), an essential component of innate immunity, are very important resources for human therapeutics to counter the current threat of drug resistance. We have previously established that one such AMP, α-melanocyte stimulating hormone (α-MSH), an endogenous neuropeptide, and its derivatives have potent antimicrobial activity against Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA). However, the immense potential of α-MSH for therapeutic development against staphylococcal infections is marred by its reduced efficacy in the presence of standard microbiological culture medium. To overcome this issue, in this study, we designed a series of five novel analogues of the C-terminal fragment of α-MSH, i.e., α-MSH(6-13), by replacing uncharged and less hydrophobic residues with tryptophan and arginine to increase the hydrophobicity and cationic charge of the peptide, respectively. While all of the peptides showed a preferential interaction with negatively charged phospholipid vesicles, the most hydrophobic and cationic peptide, i.e., Ana-5, exhibited the highest activity against S. aureus cells while maintaining cell selectivity. Moreover, Ana-5 could retain its activity even in complex media like the Mueller Hinton broth and displayed rapid bactericidal activity in the presence of serum. Ana-5 also caused rapid bacterial membrane depolarization, permeabilization, and cell lysis and was able to bind to polyanionic plasmid DNA suggesting a possible dual mode of action of the peptide. Importantly, Ana-5 was able to eradicate intracellular S. aureus in fibroblast cells similar to conventional antibiotics. Collectively, in the present study, we obtained a potent α-MSH-based analogue with excellent staphylocidal potency in microbial growth medium and ex vivo efficacy, which may translate into therapeutic application.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
