Multispectroscopic and Computational Analysis Insight into the Interaction of Cationic Diester-Bonded Gemini Surfactants with Serine Protease α-Chymotrypsin
- PMID: 32118178
- PMCID: PMC7045504
- DOI: 10.1021/acsomega.9b04142
Multispectroscopic and Computational Analysis Insight into the Interaction of Cationic Diester-Bonded Gemini Surfactants with Serine Protease α-Chymotrypsin
Abstract
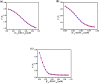
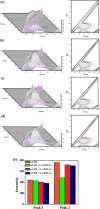
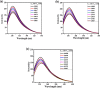
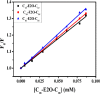
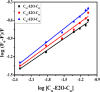
Accumulation of different protein-surfactant mixtures affords further knowledge about the structure-property interactions of biomacromolecules. They will help design suitable surfactants, which, in turn, can enhance the utilization of protein-surfactant complexes in biotechnologies, cosmetics, and food industry realms. Owing to their adaptable and remarkably notable properties, we are describing herein the interaction of C m -E2O-C m gemini surfactants (m = 12, 14, and 16) with α-CHT by employing various spectroscopic techniques including with molecular docking and density functional theory (DFT) method. Results have revealed complex formation, unfolding, and a static quenching mechanism in the interaction of gemini surfactants with α-CHT. The Stern-Volmer constant (K SV), quenching constant (k q), the number of binding sites (n), and binding constant (K b) were interrogated by utilizing the fluorescence quenching method, UV-vis, synchronous, 3-D, and resonance Rayleigh scattering fluorescence studies. The data perceive the α-CHT-C m -E2O-C m complex formation along with conformational alterations induced in α-CHT. The contribution of aromatic residues to a nonpolar environment is illustrated by pyrene fluorescence. Fourier transform infrared spectroscopy and circular dichroism outcomes reveal conformational modifications in the secondary structure of α-CHT with the permutation of gemini surfactants. The computational calculations (molecular docking and DFT) further corroborate the complex formation between α-CHT and C m -E2O-C m gemini surfactants and the contribution of electrostatic/hydrophobic interaction forces therein.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






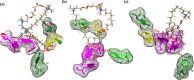


Similar articles
-
Molecular binding interaction of pyridinium based gemini surfactants with bovine serum albumin: Insights from physicochemical, multispectroscopic, and computational analysis.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Apr 5;250:119350. doi: 10.1016/j.saa.2020.119350. Epub 2020 Dec 22. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33387804
-
Solution behaviour of lysozyme in the presence of novel biodegradable gemini surfactants.Int J Biol Macromol. 2018 Oct 1;117:301-307. doi: 10.1016/j.ijbiomac.2018.05.186. Epub 2018 May 26. Int J Biol Macromol. 2018. PMID: 29807079
-
Molecular interaction of di-ester bonded cationic Gemini surfactants with pepsin: in vitro and in silico perspectives.J Biomol Struct Dyn. 2023;41(21):12276-12291. doi: 10.1080/07391102.2023.2168759. Epub 2023 Jan 25. J Biomol Struct Dyn. 2023. PMID: 36695086
-
Exploring the binding mode of ester-based cationic gemini surfactants with calf thymus DNA: A detailed physicochemical, spectroscopic and theoretical study.Bioorg Chem. 2022 Feb;119:105555. doi: 10.1016/j.bioorg.2021.105555. Epub 2021 Dec 11. Bioorg Chem. 2022. PMID: 34923244
-
Interactions of bovine serum albumin with cationic imidazolium and quaternary ammonium gemini surfactants: effects of surfactant architecture.J Colloid Interface Sci. 2013 Jan 1;389(1):175-81. doi: 10.1016/j.jcis.2012.08.067. Epub 2012 Sep 19. J Colloid Interface Sci. 2013. PMID: 23044272
Cited by
-
Comprehensive biochemical, molecular and structural characterization of subtilisin with fibrinolytic potential in bioprocessing.Bioresour Bioprocess. 2025 Mar 21;12(1):21. doi: 10.1186/s40643-025-00860-1. Bioresour Bioprocess. 2025. PMID: 40117024 Free PMC article.
-
Increasing the Efficacy of Seproxetine as an Antidepressant Using Charge-Transfer Complexes.Molecules. 2022 May 20;27(10):3290. doi: 10.3390/molecules27103290. Molecules. 2022. PMID: 35630766 Free PMC article.
-
Charge-transfer chemistry of two corticosteroids used adjunctively to treat COVID-19. Part II: The CT reaction of hydrocortisone and dexamethasone donors with TCNQ and fluoranil acceptors in five organic solvents.J Mol Liq. 2022 Oct 1;363:119878. doi: 10.1016/j.molliq.2022.119878. Epub 2022 Jul 21. J Mol Liq. 2022. PMID: 35880006 Free PMC article.
-
Multispectral and Molecular Docking Studies Reveal Potential Effectiveness of Antidepressant Fluoxetine by Forming π-Acceptor Complexes.Molecules. 2022 Sep 10;27(18):5883. doi: 10.3390/molecules27185883. Molecules. 2022. PMID: 36144618 Free PMC article.
-
Attempting to Increase the Effectiveness of the Antidepressant Trazodone Hydrochloride Drug Using π-Acceptors.Int J Environ Res Public Health. 2022 Sep 8;19(18):11281. doi: 10.3390/ijerph191811281. Int J Environ Res Public Health. 2022. PMID: 36141553 Free PMC article.
References
LinkOut - more resources
Full Text Sources

