Ancient species offers contemporary therapeutics: an update on shark VNAR single domain antibody sequences, phage libraries and potential clinical applications
- PMID: 32118195
- PMCID: PMC7034638
- DOI: 10.1093/abt/tbaa001
Ancient species offers contemporary therapeutics: an update on shark VNAR single domain antibody sequences, phage libraries and potential clinical applications
Abstract
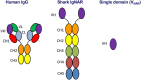
The antigen binding variable domain (VNAR) of the shark immunoglobulin new antigen receptor (IgNAR) evolved approximately 500 million years ago and it is one of the smallest antibody fragments in the animal kingdom with sizes of 12-15 kDa. This review discusses the current knowledge of the shark VNAR single domain sequences and ongoing development of shark VNARs as research tools as well as potential therapeutics, in particular highlighting the recent next-generation sequencing analysis of 1.2 million shark VNAR sequences and construction of a large phage displayed shark VNAR library from six naïve adult nurse sharks (Ginglymostoma cirratum). The large phage-displayed VNAR single domain library covers all the four known VNAR types (Types I-IV) and many previously unknown types. Ongoing preclinical development will help define the utility of shark VNAR single domains as a potentially new family of drug candidates for treating cancer and other human diseases.
Keywords: VNAR single domain; antibody engineering; next-generation sequencing; nurse shark (Ginglymostoma cirratum); phage display library.
© The Author(s) 2020. Published by Oxford University Press on behalf of Antibody Therapeutics.
Figures

References
-
- Dooley, H, Flajnik, MF. Antibody repertoire development in cartilaginous fish. Dev Comp Immunol 2006; 30: 43–56. - PubMed
-
- Frommel, D, Litman, GW, Finstad, Jet al. The evolution of the immune response. XI. The immunoglobulins of the horned shark, Heterodontus francisci: purification, characterization and structural requirement for antibody activity. J Immunol 1971; 106: 1234–43. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
