Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial
- PMID: 32119035
- PMCID: PMC7052786
- DOI: 10.1001/jamaneurol.2020.0073
Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial
Erratum in
-
Correction to Collaborator's Name.JAMA Neurol. 2020 May 1;77(5):655. doi: 10.1001/jamaneurol.2020.0749. JAMA Neurol. 2020. PMID: 32391881 Free PMC article. No abstract available.
Abstract
Importance: Clinical evidence supports effectiveness of cannabidiol for treatment-resistant seizures in Dravet syndrome, but this trial is the first to evaluate the 10-mg/kg/d dose.
Objective: To evaluate the efficacy and safety of a pharmaceutical formulation of cannabidiol, 10 and 20 mg/kg/d, vs placebo for adjunctive treatment of convulsive seizures in patients with Dravet syndrome.
Design, setting, and participants: This double-blind, placebo-controlled, randomized clinical trial (GWPCARE2) recruited patients from April 13, 2015, to November 10, 2017, with follow-up completed on April 9, 2018. Of 285 patients screened from 38 centers in the United States, Spain, Poland, the Netherlands, Australia, and Israel, 86 were excluded, and 199 were randomized. Patients were aged 2 to 18 years with a confirmed diagnosis of Dravet syndrome and at least 4 convulsive seizures during the 4-week baseline period while receiving at least 1 antiepileptic drug. Data were analyzed from November 16 (date of unblinding) to December 13 (date of final outputs), 2018, based on intention to treat and per protocol.
Interventions: Patients received cannabidiol oral solution at a dose of 10 or 20 mg/kg per day (CBD10 and CBD20 groups, respectively) or matched placebo in 2 equally divided doses for 14 weeks. All patients, caregivers, investigators, and individuals assessing data were blinded to group assignment.
Main outcomes and measures: The primary outcome was change from baseline in convulsive seizure frequency during the treatment period. Secondary outcomes included change in all seizure frequency, proportion with at least a 50% reduction in convulsive seizure activity, and change in Caregiver Global Impression of Change score.
Results: Of 198 eligible patients (mean [SD] age, 9.3 [4.4] years; 104 female [52.5%]), 66 were randomized to the CBD10 group, 67 to the CBD20 group, and 65 to the placebo group, and 190 completed treatment. The percentage reduction from baseline in convulsive seizure frequency was 48.7% for CBD10 group and 45.7% for the CBD20 group vs 26.9% for the placebo group; the percentage reduction from placebo was 29.8% (95% CI, 8.4%-46.2%; P = .01) for CBD10 group and 25.7% (95% CI, 2.9%-43.2%; P = .03) for the CBD20 group. The most common adverse events were decreased appetite, diarrhea, somnolence, pyrexia, and fatigue. Five patients in the CBD20 group discontinued owing to adverse events. Elevated liver transaminase levels occurred more frequently in the CBD20 (n = 13) than the CBD10 (n = 3) group, with all affected patients given concomitant valproate sodium.
Conclusions and relevance: Adjunctive cannabidiol at doses of 10 and 20 mg/kg/d led to similar clinically relevant reductions in convulsive seizure frequency with a better safety and tolerability profile for the 10-mg/kg/d dose in children with treatment-resistant Dravet syndrome. Dose increases of cannabidiol to greater than 10 mg/kg/d should be tailored to individual efficacy and safety.
Trial registration: ClinicalTrials.gov Identifier: NCT02224703.
Conflict of interest statement
Figures

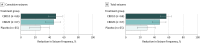
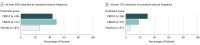
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

