Stem Cell-Derived Endothelial Cell Model that Responds to Tobacco Smoke Like Primary Endothelial Cells
- PMID: 32119531
- PMCID: PMC8020615
- DOI: 10.1021/acs.chemrestox.9b00363
Stem Cell-Derived Endothelial Cell Model that Responds to Tobacco Smoke Like Primary Endothelial Cells
Abstract
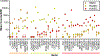
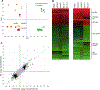
To clarify how smoking leads to heart attack and stroke, we developed an endothelial cell model (iECs) generated from human induced Pluripotent Stem Cells (iPSC) and evaluated its responses to tobacco smoke. These iECs exhibited a uniform endothelial morphology, and expressed markers PECAM1/CD31, VWF/ von Willebrand Factor, and CDH5/VE-Cadherin. The iECs also exhibited tube formation and acetyl-LDL uptake comparable to primary endothelial cells (EC). RNA sequencing (RNA-Seq) revealed a robust correlation coefficient between iECs and EC (R = 0.76), whereas gene responses to smoke were qualitatively nearly identical between iECs and primary ECs (R = 0.86). Further analysis of transcriptional responses implicated 18 transcription factors in regulating responses to smoke treatment, and identified gene sets regulated by each transcription factor, including pathways for oxidative stress, DNA damage/repair, ER stress, apoptosis, and cell cycle arrest. Assays for 42 cytokines in HUVEC cells and iECs identified 23 cytokines that responded dynamically to cigarette smoke. These cytokines and cellular stress response pathways describe endothelial responses for lymphocyte attachment, activation of coagulation and complement, lymphocyte growth factors, and inflammation and fibrosis; EC-initiated events that collectively lead to atherosclerosis. Thus, these studies validate the iEC model and identify transcriptional response networks by which ECs respond to tobacco smoke. Our results systematically trace how ECs use these response networks to regulate genes and pathways, and finally cytokine signals to other cells, to initiate the diverse processes that lead to atherosclerosis and cardiovascular disease.
Figures





References
-
- Hoffmann D, Djordjevic MV, and Hoffmann I. (1997) The changing cigarette. Prev Med 26, 427–434. - PubMed
-
- Alberg AJ, Shopland DR, and Cummings KM (2014) The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol 179, 403–412. - PMC - PubMed
-
- Messner B, and Bernhard D. (2014) Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 34, 509–515. - PubMed
-
- Leone A. (2015) Toxics of Tobacco Smoke and Cardiovascular System: From Functional to Cellular Damage. Curr Pharm Des 21, 4370–4379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

