Endothelin-1 (ET-1) promotes a proinflammatory microglia phenotype in diabetic conditions
- PMID: 32119570
- PMCID: PMC7483816
- DOI: 10.1139/cjpp-2019-0679
Endothelin-1 (ET-1) promotes a proinflammatory microglia phenotype in diabetic conditions
Abstract
Diabetes increases the risk and severity of cognitive impairment, especially after ischemic stroke. It is also known that the activation of the endothelin (ET) system is associated with cognitive impairment and microglia around the periinfarct area produce ET-1. However, little is known about the effect of ET-1 on microglial polarization, especially under diabetic conditions. We hypothesized that (i) ET-1 activates microglia to the proinflammatory M-1-like phenotype and (ii) hypoxia/ lipopolysaccharide (LPS) activates the microglial ET system and promotes microglial activation towards the M-1 phenotype in diabetic conditions. Microglial cells (C8B4) cultured under normal-glucose (25 mmol/L) conditions and diabetes-mimicking high-glucose (50 mmol/L) conditions for 48 h were stimulated with ET-1, cobalt chloride (200 μmol/L), or LPS (100 ng/mL) for 24 h. PPET-1, ET receptor subtypes, and M1/M2 marker gene mRNA expression were measured by RT-PCR. Secreted ET-1 was measured by ELISA. A high dose of ET-1 (1 μmol/L) increases the mRNA levels of ET receptors and activates the microglia towards the M1 phenotype. Hypoxia or LPS activates the ET system in microglial cells and shifts the microglia towards the M1 phenotype in diabetic conditions. These in vitro observations warrant further investigation into the role of ET-1-mediated activation of proinflammatory microglia in post-stroke cognitive impairment in diabetes.
Keywords: AVC; ET-1; brain; cellules microgliales; cerveau; diabetes; diabète; microglial cells; stroke.
Figures


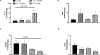
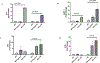
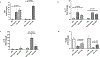
References
-
- Abdelsaid M, Kaczmarek J, Coucha M, and Ergul A. 2014a. Dual endothelin receptor antagonism with bosentan reverses established vascular remodeling and dysfunctional angiogenesis in diabetic rats: relevance to glycemic control. Life Sci 118(2): 268–273. doi: 10.1016/j.lfs.2014.01.008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

