Zimbabwe's national third-line antiretroviral therapy program: Cohort description and treatment outcomes
- PMID: 32119663
- PMCID: PMC7051055
- DOI: 10.1371/journal.pone.0228601
Zimbabwe's national third-line antiretroviral therapy program: Cohort description and treatment outcomes
Abstract
Background: In 2015, Zimbabwe introduced third-line antiretroviral therapy (ART) through four designated treatment centers; three government clinics in Harare and Bulawayo, and Newlands Clinic (NC), operated by a private voluntary organization in Harare. We describe characteristics of patients receiving third line ART and analyzed treatment outcomes in this national programme as of 31 December 2018.
Methods: We described the population using proportions for categorical variables, and medians and interquartile ranges for continuous variables. Patients from NC, where data were more complete, were followed from the date of starting third-line ART until death, transfer, loss to follow up or 31 December 2018.
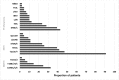
Results: A total of 209 patients had ever received third-line ART: 124 at NC and 85 from the three government clinics. HIV genotype results were available for 89 (72%) patients at NC and fourteen (16.5%) patients in the government clinics. Median duration of third line ART (years) in the government clinics was 2.3 (IQR:0.6-3.4), 1.3 (IQR: 0.7-1.7) and 1 (0.6-1.9). Of the 67 patients who received third line ART in the government clinics for at least six months, 53 (79%) had most recent viral load (VL) < 1000 copies/ml. Data on other treatment outcomes from government clinics were incomplete. From NC: a total of 109 (88%) patients were still in care, 13 (10.5%) had died and 2 (1.5%) were transferred. Median duration of third-line ART was 1.4 years (IQR: 0.6-2.8). Among the 111 NC patients who had received third-line ART for at least 6 months, 83 (75%) had a VL <50 copies/ml and 106 (95.5%) had a VL <1000 copies/ml.
Conclusion: Our findings demonstrate that, with comprehensive care, patients failing second-line ART can achieve high rates of virological suppression on third-line regimens. There is need to decentralize the provision of third-line ART in Zimbabwe. More needs to be done to improve completeness of data in the government clinics.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures
Similar articles
-
Outcomes of an HIV cohort after a decade of comprehensive care at Newlands Clinic in Harare, Zimbabwe: TENART cohort.PLoS One. 2017 Oct 24;12(10):e0186726. doi: 10.1371/journal.pone.0186726. eCollection 2017. PLoS One. 2017. PMID: 29065149 Free PMC article.
-
Treatment outcomes in HIV infected patients older than 50 years attending an HIV clinic in Harare, Zimbabwe: A cohort study.PLoS One. 2021 Jun 9;16(6):e0253000. doi: 10.1371/journal.pone.0253000. eCollection 2021. PLoS One. 2021. PMID: 34106989 Free PMC article.
-
Third-Line Antiretroviral Therapy Program in the South African Public Sector: Cohort Description and Virological Outcomes.J Acquir Immune Defic Syndr. 2019 Jan 1;80(1):73-78. doi: 10.1097/QAI.0000000000001883. J Acquir Immune Defic Syndr. 2019. PMID: 30334876 Free PMC article.
-
Second-line failure and first experience with third-line antiretroviral therapy in Mumbai, India.Glob Health Action. 2014 Jul 30;7:24861. doi: 10.3402/gha.v7.24861. eCollection 2014. Glob Health Action. 2014. PMID: 25084835 Free PMC article.
-
Genotyping and outcomes of presumptive second line ART failure cases switched to third line or maintained on second line ART in Mumbai, India.PLoS One. 2019 Nov 21;14(11):e0225631. doi: 10.1371/journal.pone.0225631. eCollection 2019. PLoS One. 2019. PMID: 31751433 Free PMC article.
Cited by
-
Risk factors for HIV virological non-suppression among adolescents with common mental disorder symptoms in Zimbabwe: a cross-sectional study.J Int AIDS Soc. 2021 Aug;24(8):e25773. doi: 10.1002/jia2.25773. J Int AIDS Soc. 2021. PMID: 34402199 Free PMC article. Clinical Trial.
-
HIV drug resistance monitoring in the era of dolutegravir and injectable long-acting cabotegravir in resource-limited settings.AIDS. 2023 Aug 1;37(10):1629-1631. doi: 10.1097/QAD.0000000000003600. AIDS. 2023. PMID: 37450629 Free PMC article. No abstract available.
-
Cost-effectiveness of screening and treating alcohol use and depression among people living with HIV in Zimbabwe: a mathematical modeling study.BMC Med. 2024 Oct 21;22(1):481. doi: 10.1186/s12916-024-03674-8. BMC Med. 2024. PMID: 39428460 Free PMC article.
-
Third-line antiretroviral therapy, including raltegravir (RAL), darunavir (DRV/r) and/or etravirine (ETR), is well tolerated and achieves durable virologic suppression over 144 weeks in resource-limited settings: ACTG A5288 strategy trial.J Int AIDS Soc. 2022 Jun;25(6):e25905. doi: 10.1002/jia2.25905. J Int AIDS Soc. 2022. PMID: 36039892 Free PMC article.
-
Virologic outcomes on dolutegravir-, atazanavir-, or efavirenz-based ART in urban Zimbabwe: A longitudinal study.PLoS One. 2024 Feb 23;19(2):e0293162. doi: 10.1371/journal.pone.0293162. eCollection 2024. PLoS One. 2024. PMID: 38394297 Free PMC article.
References
-
- WHO | Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection WHO. World Health Organization; 2016; - PubMed
-
- National Medicine and Therapeutics Policy Advisory Committee; The AIDS and TB Directorate Ministry of Health and Child Care. Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in Zimbabwe. 2013; 1–88. Available: https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUK...
-
- Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale-up of HIV viral load monitoring—seven Sub-Saharan African countries, January 2015–June 2016. Morbidity and Mortality Weekly Report. Department of Health and Human Services; 2016. pp. 1332–1335. 10.15585/mmwr.mm6547a2 - DOI - PubMed