Evolutionary analysis of Mycobacterium bovis genotypes across Africa suggests co-evolution with livestock and humans
- PMID: 32119671
- PMCID: PMC7077849
- DOI: 10.1371/journal.pntd.0008081
Evolutionary analysis of Mycobacterium bovis genotypes across Africa suggests co-evolution with livestock and humans
Abstract
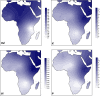
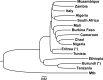
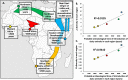
Mycobacterium bovis is the pathogenic agent responsible for bovine tuberculosis (bTB), a zoonotic disease affecting mostly cattle, but also transmittable to humans and wildlife. Genetic studies on M. bovis allow to detect possible routes of bTB transmission and the identification of genetic reservoirs that may provide an essential framework for public health action. We used a database with 1235 M. bovis genotypes collected from different regions in Africa with 45 new Mozambican samples. Our analyses, based on phylogeographic and population genetics' approaches, allowed to identify two clear trends. First, the genetic diversity of M. bovis is geographically clustered across the continent, with the only incidences of long-distance sharing of genotypes, between South Africa and Algeria, likely due to recent European introductions. Second, there is a broad gradient of diversity from Northern to Southern Africa with a diversity focus on the proximity to the Near East, where M. bovis likely emerged with animal domestication in the last 10,000 years. Diversity indices are higher in Eastern Africa, followed successively by Northern, Central, Southern and Western Africa, roughly correlating with the regional archaeological records of introduction of animal domesticates. Given this scenario M. bovis in Africa was probably established millennia ago following a concomitant spread with cattle, sheep and goat. Such scenario could translate into long-term locally adapted lineages across Africa. This work describes a novel scenario for the spread of M. bovis in Africa using the available genetic data, opening the field to further studies using higher resolution genomic data.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Tracing cross species transmission of Mycobacterium bovis at the wildlife/livestock interface in South Africa.BMC Microbiol. 2020 Mar 4;20(1):49. doi: 10.1186/s12866-020-01736-4. BMC Microbiol. 2020. PMID: 32131736 Free PMC article.
-
Evidence of increasing intra and inter-species transmission of Mycobacterium bovis in South Africa: are we losing the battle?Prev Vet Med. 2014 Jul 1;115(1-2):10-7. doi: 10.1016/j.prevetmed.2014.03.011. Epub 2014 Mar 21. Prev Vet Med. 2014. PMID: 24703246
-
Population structure and history of Mycobacterium bovis European 3 clonal complex reveal transmission across ecological corridors of unrecognized importance in Portugal.Microbiol Spectr. 2024 Jul 2;12(7):e0382923. doi: 10.1128/spectrum.03829-23. Epub 2024 May 21. Microbiol Spectr. 2024. PMID: 38771094 Free PMC article.
-
Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 1: Review of epidemiology and laboratory submissions in Great Britain 2004-2010.Vet J. 2013 Nov;198(2):339-45. doi: 10.1016/j.tvjl.2013.09.006. Epub 2013 Sep 20. Vet J. 2013. PMID: 24268485 Review.
-
Epidemiology of Mycobacterium bovis and Mycobacterium tuberculosis in animals: Transmission dynamics and control challenges of zoonotic TB in Ethiopia.Prev Vet Med. 2018 Oct 1;158:1-17. doi: 10.1016/j.prevetmed.2018.06.012. Epub 2018 Jun 28. Prev Vet Med. 2018. PMID: 30220382 Review.
Cited by
-
Integrated Bioinformatics Analysis for Target Identification and Evaluation of Recombinant Protein as an Antigen for Intradermal Skin Test in Bovine Tuberculosis Diagnosis.ACS Omega. 2025 Feb 24;10(9):9187-9196. doi: 10.1021/acsomega.4c09374. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092765 Free PMC article.
-
Molecular surveillance of tuberculosis-causing mycobacteria in wastewater.Heliyon. 2022 Feb 4;8(2):e08910. doi: 10.1016/j.heliyon.2022.e08910. eCollection 2022 Feb. Heliyon. 2022. PMID: 35198775 Free PMC article.
-
The source and fate of Mycobacterium tuberculosis complex in wastewater and possible routes of transmission.BMC Public Health. 2022 Jan 20;22(1):145. doi: 10.1186/s12889-022-12527-z. BMC Public Health. 2022. PMID: 35057793 Free PMC article.
-
Recent progress in the genotyping of bovine tuberculosis and its rapid diagnosis via nanoparticle-based electrochemical biosensors.RSC Adv. 2023 Oct 30;13(45):31795-31810. doi: 10.1039/d3ra05606f. eCollection 2023 Oct 26. RSC Adv. 2023. PMID: 37908649 Free PMC article. Review.
-
An evolutionary conserved olfactory receptor for foodborne and semiochemical alkylpyrazines.FASEB J. 2021 Jun;35(6):e21638. doi: 10.1096/fj.202100224R. FASEB J. 2021. PMID: 34047404 Free PMC article.
References
-
- WHO, FAO, OIE. Roadmap for zoonotic tuberculosis. 2017;World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE)(Available online at: http://apps.who.int/iris/bitstream/handle/10665/259229/9789241513043-eng...).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

