Polarized lung inflammation and Tie2/angiopoietin-mediated endothelial dysfunction during severe Orientia tsutsugamushi infection
- PMID: 32119672
- PMCID: PMC7067486
- DOI: 10.1371/journal.pntd.0007675
Polarized lung inflammation and Tie2/angiopoietin-mediated endothelial dysfunction during severe Orientia tsutsugamushi infection
Abstract
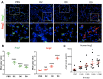
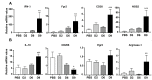
Orientia tsutsugamushi infection can cause acute lung injury and high mortality in humans; however, the underlying mechanisms are unclear. Here, we tested a hypothesis that dysregulated pulmonary inflammation and Tie2-mediated endothelial malfunction contribute to lung damage. Using a murine model of lethal O. tsutsugamushi infection, we demonstrated pathological characteristics of vascular activation and tissue damage: 1) a significant increase of ICAM-1 and angiopoietin-2 (Ang2) proteins in inflamed tissues and lung-derived endothelial cells (EC), 2) a progressive loss of endothelial quiescent and junction proteins (Ang1, VE-cadherin/CD144, occuludin), and 3) a profound impairment of Tie2 receptor at the transcriptional and functional levels. In vitro infection of primary human EC cultures and serum Ang2 proteins in scrub typhus patients support our animal studies, implying endothelial dysfunction in severe scrub typhus. Flow cytometric analyses of lung-recovered cells further revealed that pulmonary macrophages (MΦ) were polarized toward an M1-like phenotype (CD80+CD64+CD11b+Ly6G-) during the onset of disease and prior to host death, which correlated with the significant loss of CD31+CD45- ECs and M2-like (CD206+CD64+CD11b+Ly6G-) cells. In vitro studies indicated extensive bacterial replication in M2-type, but not M1-type, MΦs, implying the protective and pathogenic roles of M1-skewed responses. This is the first detailed investigation of lung cellular immune responses during acute O. tsutsugamushi infection. It uncovers specific biomarkers for vascular dysfunction and M1-skewed inflammatory responses, highlighting future therapeutic research for the control of this neglected tropical disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Type 1-skewed neuroinflammation and vascular damage associated with Orientia tsutsugamushi infection in mice.PLoS Negl Trop Dis. 2017 Jul 24;11(7):e0005765. doi: 10.1371/journal.pntd.0005765. eCollection 2017 Jul. PLoS Negl Trop Dis. 2017. PMID: 28742087 Free PMC article.
-
IL-33-Dependent Endothelial Activation Contributes to Apoptosis and Renal Injury in Orientia tsutsugamushi-Infected Mice.PLoS Negl Trop Dis. 2016 Mar 4;10(3):e0004467. doi: 10.1371/journal.pntd.0004467. eCollection 2016 Mar. PLoS Negl Trop Dis. 2016. PMID: 26943125 Free PMC article.
-
Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus.PLoS Negl Trop Dis. 2014 Aug 14;8(8):e3064. doi: 10.1371/journal.pntd.0003064. eCollection 2014 Aug. PLoS Negl Trop Dis. 2014. PMID: 25122501 Free PMC article.
-
Dysregulated Th1 Immune and Vascular Responses in Scrub Typhus Pathogenesis.J Immunol. 2018 Feb 15;200(4):1233-1240. doi: 10.4049/jimmunol.1701219. J Immunol. 2018. PMID: 29431689 Free PMC article. Review.
-
Scrub Typhus Pathogenesis: Innate Immune Response and Lung Injury During Orientia tsutsugamushi Infection.Front Microbiol. 2019 Sep 6;10:2065. doi: 10.3389/fmicb.2019.02065. eCollection 2019. Front Microbiol. 2019. PMID: 31555249 Free PMC article. Review.
Cited by
-
Intranasal Immunization With Nanoparticles Containing an Orientia tsutsugamushi Protein Vaccine Candidate and a Polysorbitol Transporter Adjuvant Enhances Both Humoral and Cellular Immune Responses.Immune Netw. 2023 Dec 15;23(6):e47. doi: 10.4110/in.2023.23.e47. eCollection 2023 Dec. Immune Netw. 2023. PMID: 38188601 Free PMC article.
-
Orientia tsutsugamushi modulates p53, the cell cycle, and genotoxicity to maintain its intracellular niche.Nat Commun. 2025 Aug 19;16(1):7728. doi: 10.1038/s41467-025-63149-z. Nat Commun. 2025. PMID: 40830139 Free PMC article.
-
Orientia tsutsugamushi selectively stimulates the C-type lectin receptor Mincle and type 1-skewed proinflammatory immune responses.PLoS Pathog. 2021 Jul 28;17(7):e1009782. doi: 10.1371/journal.ppat.1009782. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34320039 Free PMC article.
-
Distinct Role of TNFR1 and TNFR2 in Protective Immunity Against Orientia tsutsugamushi Infection in Mice.Front Immunol. 2022 Apr 11;13:867924. doi: 10.3389/fimmu.2022.867924. eCollection 2022. Front Immunol. 2022. PMID: 35479068 Free PMC article.
-
Alterations in germinal center formation and B cell activation during severe Orientia tsutsugamushi infection in mice.PLoS Negl Trop Dis. 2023 May 5;17(5):e0011090. doi: 10.1371/journal.pntd.0011090. eCollection 2023 May. PLoS Negl Trop Dis. 2023. PMID: 37146079 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

