High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation
- PMID: 32119801
- PMCID: PMC7196362
- DOI: 10.1089/met.2019.0115
High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation
Abstract
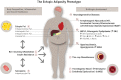
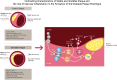
Current algorithms for assessing risk of atherosclerotic cardiovascular disease (ASCVD) and, in particular, the reliance on low-density lipoprotein (LDL) cholesterol in conditions where this measurement is discordant with apoB and LDL-particle concentrations fail to identify a sizeable part of the population at high risk for adverse cardiovascular events. This results in missed opportunities for ASCVD prevention, most notably in those with metabolic syndrome, prediabetes, and diabetes. There is substantial evidence that accumulation of ectopic fat and associated metabolic traits are markers for and pathogenic components of high-risk atherosclerosis. Conceptually, the subset of advanced lesions in high-risk atherosclerosis that triggers vascular complications is closely related to a set of coordinated high-risk traits clustering around a distinct metabolic phenotype. A key feature of this phenotype is accumulation of ectopic fat, which, coupled with age-related muscle loss, creates a milieu conducive for the development of ASCVD: atherogenic dyslipidemia, nonresolving inflammation, endothelial dysfunction, hyperinsulinemia, and impaired fibrinolysis. Sustained vascular inflammation, a hallmark of high-risk atherosclerosis, impairs plaque stabilization in this phenotype. This review describes how metabolic and inflammatory processes that are promoted in large measure by ectopic adiposity, as opposed to subcutaneous adipose tissue, relate to the pathogenesis of high-risk atherosclerosis. Clinical biomarkers indicative of these processes provide incremental information to standard risk factor algorithms and advanced lipid testing identifies atherogenic lipoprotein patterns that are below the discrimination level of standard lipid testing. This has the potential to enable improved identification of high-risk patients who are candidates for therapeutic interventions aimed at prevention of ASCVD.
Keywords: atherosclerosis; dyslipidemia; ectopic adipose tissue; inflammation; lifestyle; metabolic syndrome.
Conflict of interest statement
K.L., N.K., N.W., U.N., B.L., J.S., and O.W. declare that no competing financial interests exist with respect to this article. A.L.M. is employed by Virta Health and has been offered stock options. C.v.S. operates Omegametrix, a laboratory for fatty acid analyses. He consults for BASF/Pronova, and Huntsworth Medical, and received speaker's honoraria from Abbott, DSM, and Norsan. R.M.K. is on the Scientific Advisory Board of Virta Health and Day Two, has grant support from Quest Diagnostics and Dairy Management, Inc., and has a licensed patent for lipoprotein particle analysis by ion mobility.
Figures
References
-
- Davidson MH, Ballantyne CM, Jacobson TA, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J Clin Lipidol 2011;5:338–367 - PubMed
-
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007;116:1832–1844 - PubMed
-
- Sniderman AD, Pencina M, Thanassoulis G. ApoB. Circ Res 2019;124:1425–1427 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
