American Registry of Pathology Expert Opinions: The Spectrum of Lobular Carcinoma in Situ: Diagnostic Features and Clinical Implications
- PMID: 32120324
- PMCID: PMC7401835
- DOI: 10.1016/j.anndiagpath.2020.151481
American Registry of Pathology Expert Opinions: The Spectrum of Lobular Carcinoma in Situ: Diagnostic Features and Clinical Implications
Abstract
This review reflects a collaboration between the American Registry of Pathology (the publisher of the Armed Forces Institute of Pathology Fascicles) and Annals of Diagnostic Pathology. It is part of a series of expert recommendations on topics encountered in daily practice. The authors, 4 pathologists with expertise in breast pathology and a breast surgeon with a clinical and research interest in lobular carcinoma in situ (LCIS), met by conference call in September 2019 to develop recommendations for evaluating and reporting LCIS. Herein, we summarize the diagnostic criteria of classic LCIS and LCIS subtypes according to the most recent WHO criteria, discuss how best to distinguish LCIS from ductal carcinoma in situ in problematic cases (including the uses and limitations of E-cadherin immunohistochemistry), and review outcome and management issues for patients with LCIS.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
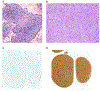
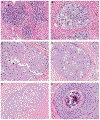


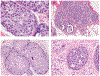

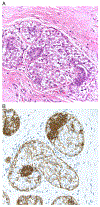
References
-
- Dabbs DJ, Schnitt SJ, Geyer FC, Weigelt B, Baehner FL, Decker T, et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol 2013;37(7):e1–11. - PubMed
-
- Canas-Marques R, Schnitt SJ. E-cadherin immunohistochemistry in breast pathology: uses and pitfalls. Histopathology. 2016;68(1):57–69. - PubMed
-
- Chen YYDT, King TA, Palacios J, Shin SJ, Simpson PT. Lobular carcinoma in situ In: Board TWCE, editor. Breast Tumours. Lyon: International Agency for Research on Cancer; 2019. p. 71–4.
-
- Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002;15(10):1044–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

