A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy
- PMID: 32120829
- PMCID: PMC7084439
- DOI: 10.3390/ijms21051619
A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy
Abstract
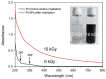
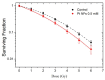
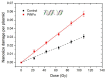
Nanomedicine has stepped into the spotlight of radiation therapy over the last two decades. Nanoparticles (NPs), especially metallic NPs, can potentiate radiotherapy by specific accumulation into tumors, thus enhancing the efficacy while alleviating the toxicity of radiotherapy. Water radiolysis is a simple, fast and environmentally-friendly method to prepare highly controllable metallic nanoparticles in large scale. In this study, we used this method to prepare biocompatible PEGylated (with Poly(Ethylene Glycol) diamine) platinum nanoflowers (Pt NFs). These nanoagents provide unique surface chemistry, which allows functionalization with various molecules such as fluorescent markers, drugs or radionuclides. The Pt NFs were produced with a controlled aggregation of small Pt subunits through a combination of grafted polymers and radiation-induced polymer cross-linking. Confocal microscopy and fluorescence lifetime imaging microscopy revealed that Pt NFs were localized in the cytoplasm of cervical cancer cells (HeLa) but not in the nucleus. Clonogenic assays revealed that Pt NFs amplify the gamma rays induced killing of HeLa cells with a sensitizing enhancement ratio (SER) of 23%, thus making them promising candidates for future cancer radiation therapy. Furthermore, the efficiency of Pt NFs to induce nanoscopic biomolecular damage by interacting with gamma rays, was evaluated using plasmids as molecular probe. These findings show that the Pt NFs are efficient nano-radio-enhancers. Finally, these NFs could be used to improve not only the performances of radiation therapy treatments but also drug delivery and/or diagnosis when functionalized with various molecules.
Keywords: cancer treatment; nanoparticle; platinum; radiation; radioenhancement; radiolysis; radiosensitization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Herold D.M., Stobbe C.C., Iyer R.V., Chapman J.D. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000;76:1357–1364. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

