Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon
- PMID: 32120871
- PMCID: PMC7084576
- DOI: 10.3390/ijerph17051519
Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon
Abstract
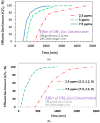
The present work investigated the application of granular activated carbon (GAC) derived from date palm pits (DPP) agricultural waste for treating gaseous ammonia. Respective findings indicate increased breakthrough time (run time at which 5% of influent ammonia is exiting with the effluent gas) with a decrease in influent ammonia and increase in GAC bed depth. At a gas flow rate of 1.1 L/min and GAC column length of 8 cm, the following breakthrough trend was noted: 1295 min (2.5 ppmv) > 712 min (5 ppmv) > 532 min (7.5 ppmv). A qualitatively similar trend was also noted for the exhaustion time results (run time at which 95% of influent ammonia is exiting with the effluent gas). The Fourier Transform Infrared Spectroscopy (FTIR) findings for the produced GAC indicated some salient functional groups at the produced GAC surface including O-H, C-H, C-O, and S=O groups. Ammonia adsorption was suggested to result from its interaction with the respective surface functional groups via different mechanisms. Comparison with a commercial GAC showed the date palm pits based GAC to be having slightly higher breakthrough and exhaustion capacity.
Keywords: activated carbon; adsorption; ammonia gas; date palm pits.
Conflict of interest statement
The author declares no conflict of interest.
Figures






References
-
- Iyobe T., Asada T., Kawata K., Oikawa K. Comparison of removal efficiencies for ammonia and amine gases between woody charcoal and activated carbon. J. Health Sci. 2004;50:148–153.
-
- Chou M.-S., Wang C.-H. Treatment of Ammonia in Air Stream by Biotrickling Filter. Aerosol Air Qual. Res. 2007;7:17–32. doi: 10.4209/aaqr.2006.09.0014. - DOI
-
- Manakasettharn S., Takahashi A., Kawamoto T., Noda K., Sugiyama Y., Nakamura T. Differences in NH3 gas adsorption behaviors of metal-hexacyanoferrate nanoparticles (Mx[FeII(CN)6]y·zH2O:M = In3+, Fe3+, and Mn2+) J. Solid State Chem. 2019;270:112–117. doi: 10.1016/j.jssc.2018.10.026. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

