Telomeres and Telomere Length: A General Overview
- PMID: 32121056
- PMCID: PMC7139734
- DOI: 10.3390/cancers12030558
Telomeres and Telomere Length: A General Overview
Abstract
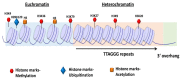
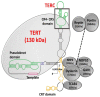
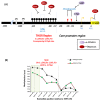
Telomeres are highly conserved tandem nucleotide repeats that include proximal double-stranded and distal single-stranded regions that in complex with shelterin proteins afford protection at chromosomal ends to maintain genomic integrity. Due to the inherent limitations of DNA replication and telomerase suppression in most somatic cells, telomeres undergo age-dependent incremental attrition. Short or dysfunctional telomeres are recognized as DNA double-stranded breaks, triggering cells to undergo replicative senescence. Telomere shortening, therefore, acts as a counting mechanism that drives replicative senescence by limiting the mitotic potential of cells. Telomere length, a complex hereditary trait, is associated with aging and age-related diseases. Epidemiological data, in general, support an association with varying magnitudes between constitutive telomere length and several disorders, including cancers. Telomere attrition is also influenced by oxidative damage and replicative stress caused by genetic, epigenetic, and environmental factors. Several single nucleotide polymorphisms at different loci, identified through genome-wide association studies, influence inter-individual variation in telomere length. In addition to genetic factors, environmental factors also influence telomere length during growth and development. Telomeres hold potential as biomarkers that reflect the genetic predisposition together with the impact of environmental conditions and as targets for anti-cancer therapies.
Keywords: TERT promoter mutations; cancer-risk; end replication; genetic variants; shelterin complex; telomere length heritability; telomere maintenance mechanisms; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gomes N.M., Ryder O.A., Houck M.L., Charter S.J., Walker W., Forsyth N.R., Austad S.N., Venditti C., Pagel M., Shay J.W., et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

