Protein Aggregation is Associated with Acinetobacter baumannii Desiccation Tolerance
- PMID: 32121206
- PMCID: PMC7142981
- DOI: 10.3390/microorganisms8030343
Protein Aggregation is Associated with Acinetobacter baumannii Desiccation Tolerance
Abstract
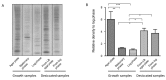
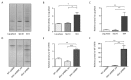
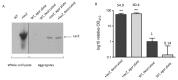
Desiccation tolerance has been implicated as an important characteristic that potentiates the spread of the bacterial pathogen Acinetobacter baumannii on dry surfaces. Here we explore several factors influencing desiccation survival of A. baumannii. At the macroscale level, we find that desiccation tolerance is influenced by cell density and growth phase. A transcriptome analysis indicates that desiccation represents a unique state for A. baumannii compared to commonly studied growth phases and strongly influences pathways responsible for proteostasis. Remarkably, we find that an increase in total cellular protein aggregates, which is often considered deleterious, correlates positively with the ability of A. baumannii to survive desiccation. We show that inducing protein aggregate formation prior to desiccation increases survival and, importantly, that proteins incorporated into cellular aggregates can retain activity. Our results suggest that protein aggregates may promote desiccation tolerance in A. baumannii through preserving and protecting proteins from damage during desiccation until rehydration occurs.
Keywords: Acinetobacter baumannii; desiccation tolerance; protein aggregation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rodríguez-Baño J., Cisneros J.M., Fernández-Cuenca F., Ribera A., Vila J., Pascual A., Martínez-Martínez L., Bou G., Pachón J., Grupo de Estudio de Infección Hospitalaria (GEIH) Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect. Control Hosp. Epidemiol. 2004;25:819–824. doi: 10.1086/502302. - DOI - PubMed
-
- Rodríguez-Baño J., García L., Ramírez E., Martínez-Martínez L., Muniain M.A., Fernández-Cuenca F., Beltrán M., Gálvez J., Rodríguez J.M., Velasco C., et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am. J. Infect. Control. 2009;37:715–722. doi: 10.1016/j.ajic.2009.01.008. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

