LPIAT, a lyso-Phosphatidylinositol Acyltransferase, Modulates Seed Germination in Arabidopsis thaliana through PIP Signalling Pathways and is Involved in Hyperosmotic Response
- PMID: 32121266
- PMCID: PMC7084726
- DOI: 10.3390/ijms21051654
LPIAT, a lyso-Phosphatidylinositol Acyltransferase, Modulates Seed Germination in Arabidopsis thaliana through PIP Signalling Pathways and is Involved in Hyperosmotic Response
Abstract
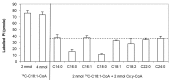
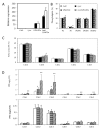
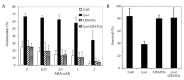
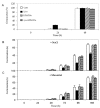
Lyso-lipid acyltransferases are enzymes involved in various processes such as lipid synthesis and remodelling. Here, we characterized the activity of an acyltransferase from Arabidopsis thaliana (LPIAT). In vitro, this protein, expressed in Escherichia coli membrane, displayed a 2-lyso-phosphatidylinositol acyltransferase activity with a specificity towards saturated long chain acyl CoAs (C16:0- and C18:0-CoAs), allowing the remodelling of phosphatidylinositol. In planta, LPIAT gene was expressed in mature seeds and very transiently during seed imbibition, mostly in aleurone-like layer cells. Whereas the disruption of this gene did not alter the lipid composition of seed, its overexpression in leaves promoted a strong increase in the phosphatidylinositol phosphates (PIP) level without affecting the PIP2 content. The spatial and temporal narrow expression of this gene as well as the modification of PIP metabolism led us to investigate its role in the control of seed germination. Seeds from the lpiat mutant germinated faster and were less sensitive to abscisic acid (ABA) than wild-type or overexpressing lines. We also showed that the protective effect of ABA on young seedlings against dryness was reduced for lpiat line. In addition, germination of lpiat mutant seeds was more sensitive to hyperosmotic stress. All these results suggest a link between phosphoinositides and ABA signalling in the control of seed germination.
Keywords: ABA; Acyltransferase; aleurone-like cells; hyperosmotic stress; phosphoinositides; seed germination.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- Tejos R., Sauer M., Vanneste S., Palacios-Gomez M., Li H., Heilmann M., van Wijk R., Vermeer J.E.M., Heilmann I., Munnik T., et al. Bipolar Plasma Membrane Distribution of Phosphoinositides and Their Requirement for Auxin-Mediated Cell Polarity and Patterning in Arabidopsis. Plant Cell. 2014;26:2114–2128. doi: 10.1105/tpc.114.126185. - DOI - PMC - PubMed
-
- Nováková P., Hirsch S., Feraru E., Tejos R., van Wijk R., Viaene T., Heilmann M., Lerche J., De Rycke R., Feraru M.I., et al. SAC phosphoinositide phosphatases at the tonoplast mediate vacuolar function in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2014;111:2818–2823. doi: 10.1073/pnas.1324264111. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

