A Preclinical Embryonic Zebrafish Xenograft Model to Investigate CAR T Cells In Vivo
- PMID: 32121414
- PMCID: PMC7139560
- DOI: 10.3390/cancers12030567
A Preclinical Embryonic Zebrafish Xenograft Model to Investigate CAR T Cells In Vivo
Abstract
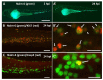
Chimeric antigen receptor (CAR) T cells have proven to be a powerful cellular therapy for B cell malignancies. Massive efforts are now being undertaken to reproduce the high efficacy of CAR T cells in the treatment of other malignancies. Here, predictive preclinical model systems are important, and the current gold standard for preclinical evaluation of CAR T cells are mouse xenografts. However, mouse xenograft assays are expensive and slow. Therefore, an additional vertebrate in vivo assay would be beneficial to bridge the gap from in vitro to mouse xenografts. Here, we present a novel assay based on embryonic zebrafish xenografts to investigate CAR T cell-mediated killing of human cancer cells. Using a CD19-specific CAR and Nalm-6 leukemia cells, we show that live observation of killing of Nalm-6 cells by CAR T cells is possible in zebrafish embryos. Furthermore, we applied Fiji macros enabling automated quantification of Nalm-6 cells and CAR T cells over time. In conclusion, we provide a proof-of-principle study that embryonic zebrafish xenografts can be used to investigate CAR T cell-mediated killing of tumor cells. This assay is cost-effective, fast, and offers live imaging possibilities to directly investigate CAR T cell migration, engagement, and killing of effector cells.
Keywords: CAR T cells; CD19 CAR; in vivo imaging; zebrafish xenografts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

