Blood Eosinophilia is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA
- PMID: 32121531
- PMCID: PMC7150785
- DOI: 10.3390/pharmaceutics12030210
Blood Eosinophilia is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA
Abstract
Background: The approvals of immune checkpoint inhibitors for several cancer types and the rapidly growing recognition that T cell-based immunotherapy significantly improves outcomes for cancer patients led to a re-emergence of cancer vaccines, including dendritic cell (DC)-based immunotherapy. Blood and tissue biomarkers to identify responders and long-term survivors and to optimize cost and cost-effectiveness of treatment are greatly needed. We wanted to investigate whether blood eosinophilia is a predictive biomarker for patients with solid tumors receiving vaccinations with DCs loaded with autologous tumor-RNA.
Methods: In total, 67 patients with metastatic solid tumors, who we treated with autologous monocyte-derived DCs transfected with total tumor mRNA, were serially analyzed for eosinophil counts and survival over the course of up to 14 years. Eosinophilic counts were performed on peripheral blood smears.
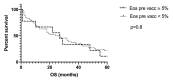
Results: Up to 87% of the patients treated with DC-based immunotherapy experienced at least once an eosinophilia of ≥ 5% after initiation of therapy; 61 % reached levels of ≥ 10% eosinophils, and 13% of patients showed eosinophil counts of 20% or above. While prevaccination eosinophil levels were not associated with survival, patients with blood eosinophilia at any point after initiation of DC-based immunotherapy showed a trend towards longer survival. There was a statistically significant difference for the patients with eosinophil counts of 20% or more (p = 0.03). In those patients, survival was prolonged to a median of 58 months (range 2-111 months), compared to a median of 20 months (range 0-119 months) in patients with lower eosinophil counts. In 12% of the patients, an immediate increase in eosinophil count of at least 10 percentage points could be detected after the first vaccine, which also appeared to correlate with survival (65 vs. 24 months; p = 0.06).
Conclusion: Blood eosinophilia appears to be an early, on-therapy biomarker in patients with solid tumors undergoing vaccination with RNA-transfected DC, specifically autologous tumor mRNA-transfected DC vaccines, and it correlates with long-term patient outcome. Eosinophilia should be systematically investigated in future trials.
Keywords: biomarkers; dendritic cell vaccines; eosinophils.
Conflict of interest statement
A.M. received travel grants or speaker fees from Abbvie, Almirall, Bristol-Myers Squibb, Pfizer, and Roche. G.S. and B.S.T. hold various patents related to DC vaccination. The companies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The other authors declare no potential conflicts of interest.
Figures




Similar articles
-
Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy.Immunotherapy. 2017 Jan;9(2):115-121. doi: 10.2217/imt-2016-0138. Immunotherapy. 2017. PMID: 28128709
-
Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD.Int J Chron Obstruct Pulmon Dis. 2016 Jul 1;11:1495-504. doi: 10.2147/COPD.S100338. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27445469 Free PMC article.
-
Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy.J Thorac Dis. 2021 May;13(5):2716-2727. doi: 10.21037/jtd-20-3525. J Thorac Dis. 2021. PMID: 34164164 Free PMC article.
-
The Role of Eosinophils in Immunotherapy.Curr Allergy Asthma Rep. 2020 Jan 7;20(1):1. doi: 10.1007/s11882-020-0895-x. Curr Allergy Asthma Rep. 2020. PMID: 31912246 Free PMC article. Review.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
Cited by
-
The use of RNA-based treatments in the field of cancer immunotherapy.Mol Cancer. 2023 Jul 7;22(1):106. doi: 10.1186/s12943-023-01807-w. Mol Cancer. 2023. PMID: 37420174 Free PMC article. Review.
-
Combining chemotherapy and autologous peptide-pulsed dendritic cells provides survival benefit in stage IV melanoma patients.J Dtsch Dermatol Ges. 2020 Nov;18(11):1270-1277. doi: 10.1111/ddg.14334. Epub 2020 Nov 16. J Dtsch Dermatol Ges. 2020. PMID: 33197129 Free PMC article.
-
Immunity and Breast Cancer: Focus on Eosinophils.Biomedicines. 2021 Aug 26;9(9):1087. doi: 10.3390/biomedicines9091087. Biomedicines. 2021. PMID: 34572273 Free PMC article. Review.
-
Eosinophils and melanoma: Implications for immunotherapy.Pigment Cell Melanoma Res. 2022 Mar;35(2):192-202. doi: 10.1111/pcmr.13025. Epub 2022 Jan 18. Pigment Cell Melanoma Res. 2022. PMID: 34927354 Free PMC article. Review.
References
-
- Uslu U., Erdmann M., Wiesinger M., Schuler G., Schuler-Thurner B. Automated Good Manufacturing Practice-compliant generation of human monocyte-derived dendritic cells from a complete apheresis product using a hollow-fiber bioreactor system overcomes a major hurdle in the manufacture of dendritic cells for cancer vaccines. Cytotherapy. 2019;21:1166–1178. doi: 10.1016/j.jcyt.2019.09.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources

