HSP70 Multi-Functionality in Cancer
- PMID: 32121660
- PMCID: PMC7140411
- DOI: 10.3390/cells9030587
HSP70 Multi-Functionality in Cancer
Abstract
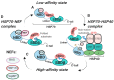
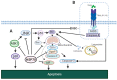
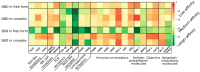
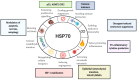
The 70-kDa heat shock proteins (HSP70s) are abundantly present in cancer, providing malignant cells selective advantage by suppressing multiple apoptotic pathways, regulating necrosis, bypassing cellular senescence program, interfering with tumor immunity, promoting angiogenesis and supporting metastasis. This direct involvement of HSP70 in most of the cancer hallmarks explains the phenomenon of cancer "addiction" to HSP70, tightly linking tumor survival and growth to the HSP70 expression. HSP70 operates in different states through its catalytic cycle, suggesting that it can multi-function in malignant cells in any of these states. Clinically, tumor cells intensively release HSP70 in extracellular microenvironment, resulting in diverse outcomes for patient survival. Given its clinical significance, small molecule inhibitors were developed to target different sites of the HSP70 machinery. Furthermore, several HSP70-based immunotherapy approaches were assessed in clinical trials. This review will explore different roles of HSP70 on cancer progression and emphasize the importance of understanding the flexibility of HSP70 nature for future development of anti-cancer therapies.
Keywords: HSP70; apoptosis; authophagy; cancer; exosomes; immunotherapy; metastasis; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Cell. Mol. Life Sci. 1962;18:571–573. doi: 10.1007/BF02172188. - DOI
-
- Moran L., Mirault M.-E., Arrigo A.P., Goldschmidt-Clermont M., Tissières A. Heat shock of Drosophila melanogaster induces the synthesis of new messenger RNAs and proteins. Philos. Trans. R. Soc. B Biol. Sci. 1978;283:391–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

