Distribution of Cadherin in the Parahippocampal Area of Developing Domestic Chicken Embryos
- PMID: 32122105
- PMCID: PMC7075654
- DOI: 10.5607/en.2020.29.1.11
Distribution of Cadherin in the Parahippocampal Area of Developing Domestic Chicken Embryos
Abstract
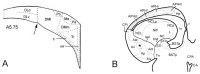
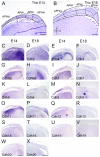
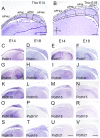
Hippocampal formation is important in spatial learning and memory. Members of the cadherin superfamily are observed in the neural system with diverse spatial and temporal expression patterns and are involved in many biological processes. To date, the avian hippocampal formation is not well understood. In this study, we examined the expression of cadherin mRNA in chicken and mouse brains to investigate the morphological and cytoarchitectural bases of hippocampal formation. Profiles of the spatiotemporal expression of cadherin mRNAs in the developing chicken embryonic parahippocampal area (APH) are provided, and layer-specific expression and spatiotemporal expression were observed in different subdivisions of the APH. That fact that some cadherins (Cdh2, Cdh8, Pcdh8 and Pcdh10) showed conserved regional expression both in the hippocampus and entorhinal cortex of mice and the hippocampal formation of chickens partially confirmed the structural homology proposed by previous scientists. This study indicates that some cadherins can be used as special markers of the avian hippocampal formation.
Keywords: Avian; Cadherin; Chicken embryos; Hippocampus; Parahippocampal area.
Figures






Similar articles
-
Differential Spatiotemporal Expression of Type I and Type II Cadherins Associated With the Segmentation of the Central Nervous System and Formation of Brain Nuclei in the Developing Mouse.Front Mol Neurosci. 2021 Mar 23;14:633719. doi: 10.3389/fnmol.2021.633719. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33833667 Free PMC article.
-
Inversion of layer-specific cadherin expression profiles and maintenance of cytoarchitectonic areas in the allocortex of the reeler mutant mouse.J Comp Neurol. 2014 Sep 1;522(13):3106-19. doi: 10.1002/cne.23572. Epub 2014 Apr 8. J Comp Neurol. 2014. PMID: 24591110
-
Calcium-binding proteins, neuronal nitric oxide synthase, and GABA help to distinguish different pallial areas in the developing and adult chicken. I. Hippocampal formation and hyperpallium.J Comp Neurol. 2006 Aug 10;497(5):751-71. doi: 10.1002/cne.21004. J Comp Neurol. 2006. PMID: 16786551
-
Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways.Hippocampus. 2000;10(4):398-410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. Hippocampus. 2000. PMID: 10985279 Review.
-
Cadherins and neuropsychiatric disorders.Brain Res. 2012 Aug 27;1470:130-44. doi: 10.1016/j.brainres.2012.06.020. Epub 2012 Jul 2. Brain Res. 2012. PMID: 22765916 Review.
Cited by
-
Dissociation of spatial and object memory in the hippocampal formation of Japanese quail.iScience. 2022 Jan 22;25(2):103805. doi: 10.1016/j.isci.2022.103805. eCollection 2022 Feb 18. iScience. 2022. PMID: 35243216 Free PMC article.
References
-
- Kang S, Lee S, Kim J, Kim JC, Kim SH, Son Y, Shin T, Youn B, Kim JS, Wang H, Yang M, Moon C. Chronic treatment with combined chemotherapeutic agents affects hippocampal micromorphometry and function in mice, independently of neuroinflammation. Exp Neurobiol. 2018;27:419–436. doi: 10.5607/en.2018.27.5.419. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

