Platelet Inhibition, Endothelial Function, and Clinical Outcome in Patients Presenting With ST-Segment-Elevation Myocardial Infarction Randomized to Ticagrelor Versus Prasugrel Maintenance Therapy: Long-Term Follow-Up of the REDUCE-MVI Trial
- PMID: 32122216
- PMCID: PMC7335553
- DOI: 10.1161/JAHA.119.014411
Platelet Inhibition, Endothelial Function, and Clinical Outcome in Patients Presenting With ST-Segment-Elevation Myocardial Infarction Randomized to Ticagrelor Versus Prasugrel Maintenance Therapy: Long-Term Follow-Up of the REDUCE-MVI Trial
Abstract
Background Off-target properties of ticagrelor might reduce microvascular injury and improve clinical outcome in patients with ST-segment-elevation myocardial infarction. The REDUCE-MVI (Evaluation of Microvascular Injury in Revascularized Patients with ST-Segment-Elevation Myocardial Infarction Treated With Ticagrelor Versus Prasugrel) trial reported no benefit of ticagrelor regarding microvascular function at 1 month. We now present the follow-up data up to 1.5 years. Methods and Results We randomized 110 patients with ST-segment-elevation myocardial infarction to either ticagrelor 90 mg twice daily or prasugrel 10 mg once a day. Platelet inhibition and peripheral endothelial function measurements including calculation of the reactive hyperemia index and clinical follow-up were obtained up to 1.5 years. Major adverse clinical events and bleedings were scored. An intention to treat and a per-protocol analysis were performed. There were no between-group differences in platelet inhibition and endothelial function. At 1 year the reactive hyperemia index in the ticagrelor group was 0.66±0.26 versus 0.61±0.28 in the prasugrel group (P=0.31). Platelet inhibition was lower at 1 month versus 1 year in the total study population (61% [42%-81%] versus 83% [61%-95%]; P<0.001), and per-protocol platelet inhibition was higher in patients randomized to ticagrelor versus prasugrel at 1 year (91% [83%-97%] versus 82% [65%-92%]; P=0.002). There was an improvement in intention to treat endothelial function in patients randomized to ticagrelor (P=0.03) but not in patients randomized to prasugrel (P=0.88). Major adverse clinical events (10% versus 14%; P=0.54) and bleedings (47% versus 63%; P=0.10) were similar in the intention-to-treat analysis in both groups. Conclusions Platelet inhibition at 1 year was higher in the ticagrelor group, without an accompanying increase in bleedings. Endothelial function improved over time in ticagrelor patients, while it did not change in the prasugrel group. Clinical Trial Registration URL: https://www.clinicaltrials.gov/. Unique Identifier: NCT02422888.
Keywords: ST‐segment‐elevation myocardial infarction; endothelial function; microvascular injury; platelet inhibition; prasugrel; ticagrelor.
Figures


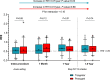
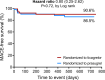
Comment in
-
Long-Term Ticagrelor Versus Prasugrel Pharmacodynamics in Patients With ST-Segment-Elevation Myocardial Infarction.J Am Heart Assoc. 2020 Mar 3;9(5):e015726. doi: 10.1161/JAHA.120.015726. Epub 2020 Mar 3. J Am Heart Assoc. 2020. PMID: 32122217 Free PMC article. No abstract available.
References
-
- Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. [2018 ESC/EACTS Guidelines on myocardial revascularization]. Eur Heart J. 2018;76:1585–1664. - PubMed
-
- Teunissen PF, de Waard GA, Hollander MR, Robbers LF, Danad I, Biesbroek PS, Amier RP, Echavarria‐Pinto M, Quiros A, Broyd C, Heymans MW, Nijveldt R, Lammertsma AA, Raijmakers PG, Allaart CP, Lemkes JS, Appelman YE, Marques KM, Bronzwaer JG, Horrevoets AJ, van Rossum AC, Escaned J, Beek AM, Knaapen P, van Royen N. Doppler‐derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2015;8:e001786. - PubMed
-
- de Waard GA, Fahrni G, de Wit D, Kitabata H, Williams R, Patel N, Teunissen PF, van de Ven PM, Umman S, Knaapen P, Perera D, Akasaka T, Sezer M, Kharbanda RK, van Royen N; Oxford Acute Myocardial Infarction Study investigators . Hyperaemic microvascular resistance predicts clinical outcome and microvascular injury after myocardial infarction. Heart. 2018;104:127–134. - PubMed
-
- Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C, Camoin‐Jau L, Paganelli F, Dignat‐George F, Guieu R. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63:872–877. - PubMed
-
- Cattaneo M, Schulz R, Nylander S. Adenosine‐mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63:2503–2509. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

